Composition by Percent Mass
MCAT General Chemistry Chapter 1 - Section 1.4 - Stoichiometry
- Home
- »
- MCAT Masterclass
- »
- Chemical and Physical Foundations of Biological Systems
- »
- General Chemistry
- »
- Stoichiometry
- »
- Chemical Formula
- »
- Composition by Percent Mass – MCAT General Chemistry
Sample MCAT Question - Composition by Percent Mass
What is the approximate percent mass of nitrogen in nitric acid?
a) 22%
b) 32%
c) 45%
d) 58%
A is correct. 22%.
The percent mass of nitrogen in nitric acid (HNO3) can be found by dividing the molar mass of nitrogen (14 g/mol) over the total molecular weight of the molecule, HNO3 (1 + 14 + 3 x 16 = 63 g/mol). If we simplify the math to 15/60, we will get 0.25, or 25%. The closest answer choice is A.
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Composition by Percent Mass
Composition by percent mass is the relative amount of the mass of a molecule represented by each constituent element within the molecule. Let’s consider as an example the percent mass of perchloric acid, HClO4. From our previous review of molecular formula, we can say that perchloric acid will consist of one hydrogen (with a molar mass of 1 g/mol), one chlorine (35 g/mol), and four oxygens (each with a molar mass of 16 g/mol, or 64 g/mol altogether). Therefore, the total molar mass of perchloric acid will be approximately 100 g/mol, as demonstrated below:
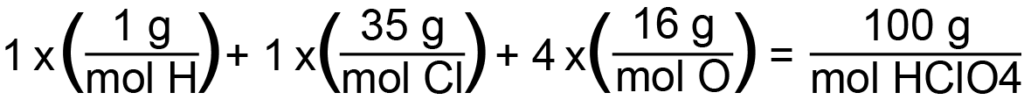
To calculate the percent mass of each substituent element, we simply divide its molar mass by the molar mass of the compound. Hydrogen, therefore, would have a percent mass of 1% (1 g/mol divided by 100 g/mol), chlorine would have a percent mass of 35% (35 g/mol divided by 100 g/mol), and oxygen a percent mass of 64% (64 g/mol divided by 100 g/mol). This gives us the relative amount of each element present in a given sample. For example, in a 10 gram sample of perchloric acid, we would find 0.1 gram of hydrogen, 3.5 grams of chlorine, and 6.4 grams of oxygen. This is especially useful in decomposition reactions, and can be used to determine the original molecular formula of an unknown molecule based on the relative mass of the decomposed elements that are measured
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.