Limiting Reactants and Theoretical Yield
MCAT General Chemistry Chapter 1 - Section 2.3 - Stoichiometry - Chemical Equations
- Home
- »
- MCAT Masterclass
- »
- Chemical and Physical Foundations of Biological Systems
- »
- General Chemistry
- »
- Stoichiometry
- »
- Chemical Equations
- »
- Limiting Reactants and Theoretical Yield – MCAT General Chemistry
Sample MCAT Question - Limiting Reactants and Theoretical Yield
a) HCl is the limiting reactant and 4.5 g Mn will remain.
b) HCl is the limiting reactant and 2.75 g Mn will remain.
c) Mn is the limiting reactant and 0.50 mol HCl will remain.
d) Mn is the limiting reactant and 1.0 mol HCl will remain.
C is correct. Mn is the limiting reactant and 0.50 mol HCl will remain.
The chemical equation that describes this reaction is as follows: Mn + HCl --> MnCl5 + H2. We need to balance this equation, so let's start by balancing the chlorines and placing a 5 as the coefficient in front of HCl: Mn + 5HCl --> MnCl5 + H2. We then need to balance the hydrogens, and the only way to do so is to put a coefficient of 10 in front of HCl and a coefficient of 5 in front of H2: Mn + 10HCl --> MnCl5 + 5H2. Lastly, we need to balance manganese atoms and the chlorines again (balancing the hydrogens unbalanced our chlorines!), so let's place a 2 as the coefficient for Mn and MnCl5: 2Mn + 10HCl --> 2MnCl5 + 5H2. To see which reagent is the limiting reagent, first calculate how many moles of each reagent we have: 5.5 g Mn / 55 g/mol Mn = 0.10 mol Mn, 200 mL * (1 L / 1000 mL) * 5 M = 1.0 mol HCl. Since HCl reacts with Mn in a 5:1 ratio, if we have 1.0 mol of HCl, we need at least 1 mol HCl * (2 mol Mn / 10 mol HCl) = 0.20 mol Mn for Mn to not be the limiting reagent. Since we have only have 0.10 mol, we know that manganese is indeed the limiting reagent. The amount of HCl that is used in the reaction is 0.10 mol Mn * (10 mol HCl / 2 mol Mn) = 0.50 mol HCl. Therefore, 1.0 mol HCl - 0.50 mol HCl = 0.50 mol HCl remains.
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
What Are Limiting Reactants?
In real-world chemical reactions, the relative ratios of reactants do not typically exactly match the stoichiometric ratios in balanced chemical equations. In these instances, one reactant will be entirely consumed when some amount of other reactants are still remaining, halting the reaction. The reactant that will be the first to be consumed completely is called the limiting reagent.
C3H8 + 5O2 → 3CO2 + 4H2O
For example, in the above reaction, if only three moles of oxygen were reacted with one mole of propane, the reaction would halt after the three moles of oxygen were consumed, with some amount of propane (specifically, 2/5th of a mole of propane) left over. The amount of carbon dioxide and water produced would be determined by the amount of propane that actually reacted and not by the amount that was provided.
What is Theoretical Yield
Practice Example: Limiting Reactants and Theoretical Yield
Let’s combine the concepts of limiting reagent and theoretical yield in an example question.
Example: What is the limiting reagent in a reaction where 66 g of propane is reacted with 256 g of oxygen? If 150 g of carbon dioxide is formed from the reaction, what is the percent yield of the reaction?
C3H8 + 5O2 → 3CO2 +4H2O
We will begin by determining the limiting reagent. From the balanced reaction, we can see that five moles of oxygen are consumed for every one mole of propane. However, this does not mean that oxygen will necessarily be consumed first. In this case, propane will be the limiting reagent if there is more than five times as much oxygen as propane. On the contrary, if there is less than five times as much oxygen as propane, then oxygen will be the limiting reagent. However, as we have discussed before, we cannot directly compare the mass of the two compounds, but must instead convert to a relatable quantity, such as moles. Looking at the periodic table, we can calculate propane’s molar mass to be 44 grams per mole, and elemental oxygen’s to be 32 grams per mole. Since we began with 66 grams of propane, this will convert to 1.5 moles of propane. Likewise, for oxygen, we will divide 256 grams of oxygen by 32 grams of oxygen per mole of oxygen, giving us a total of 8 moles of oxygen. As we mentioned earlier, propane will be the limiting reagent if there is more than five times as much oxygen as propane. That would equate to 7.5 moles of oxygen. Since we have 8 moles of oxygen, propane will be our limiting reagent, and some amount of excess oxygen (0.5 moles) will remain after all of our propane has been consumed.
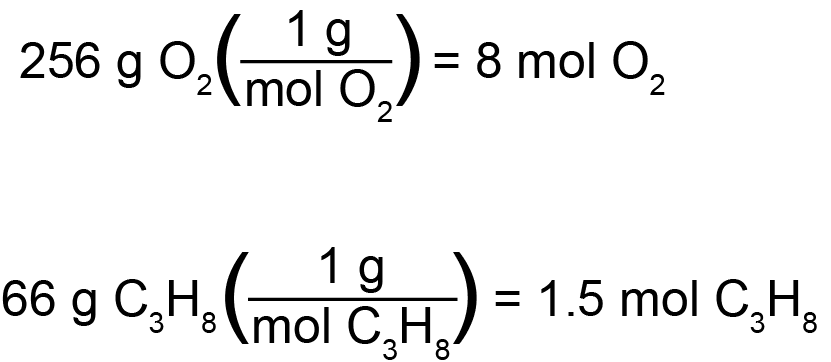
On test day, there is another simple trick to determining limiting reagent quickly. After calculating the number of moles of each reactant, simply divide the number of moles of each reactant by its coefficient: the smaller resulting number is the limiting reagent. So, in this case, 1.5 moles of propane divided by propane’s coefficient of 1 is equal to 1.5. On the other hand, 8 moles of oxygen divided by oxygen’s coefficient of 5 is equal to 1.6. 1.5 is the smaller number, and therefore propane is the limiting reagent.
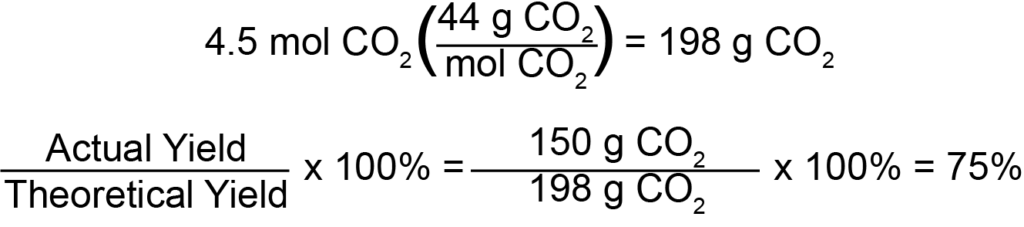
To do the next step of the question, and calculate percent yield, we must first determine the theoretical yield. This is taken by determining the moles of product created, and then converting that value to a mass value. Remember from our first question that the limiting reagent was propane, and that 1.5 moles of propane reacted. Since there is a stoichiometric ratio of 1 mole of propane for every 3 moles of carbon dioxide, the total quantity of carbon dioxide produced will be equal to 4.5 moles. To convert to a mass value, we will multiply by carbon dioxide’s molar mass (44 g/mol), giving us a final theoretical yield of 198 grams of CO2, which remember notes the theoretical, ideal maximum amount of carbon dioxide that could be produced by the reaction. To find the percent yield, we will then divide the actual yield, given to us as 150 grams, by the theoretical yield of 198 grams, for an approximate theoretical yield of 0.75, or 75%.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.