Amino Acid Classification
MCAT Biochemistry Chapter 1 - Section 1.2 - Amino Acids - Amino Acid Classification
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Amino Acids
- »
- Amino Acid Classification – MCAT Biochemistry
Sample MCAT Question - Amino Acid Classification
a) H
b) D
c) K
d) R
B is correct. D or aspartic acid.
Acidic amino acids have low side chain pKa values. Aspartic acid (one letter code: D) is an acidic amino acid and has a side chain pKa value of 3.7 (which is lower than 5). Answer choice A is incorrect because histidine (one letter code: H) is a basic amino acid and has a side chain pKa value of 6 (which is higher than 5). Answer choice C is incorrect because lysine (one letter code: K) is a basic amino acid and has a side chain pKa value of 10.7 (which is higher than 5). Answer choice D is incorrect because arginine (one letter code: R) is a basic amino acid and has a side chain pKa value of 12.1 (which is higher than 5).
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Amino Acid Classification
Amino acid classification is a very high yield MCAT topic. You should know the basic structure and 3D configuration of amino acids, their one and three-letter codes, and the pKa values of the R groups of acidic/basic amino acids. You should also be able to recognize the 20 different amino acids by their side chains. Subtleties in their side chain structures have significant implications for their behavior, and it is often beneficial to be completely familiar with them for this reason. To make this task less intimidating and more systematic, we will be dividing up the amino acids into several classes with highly similar properties. These classes include acidic, basic, polar, nonpolar, sulfur-containing, aromatic, and hydroxyl-containing.
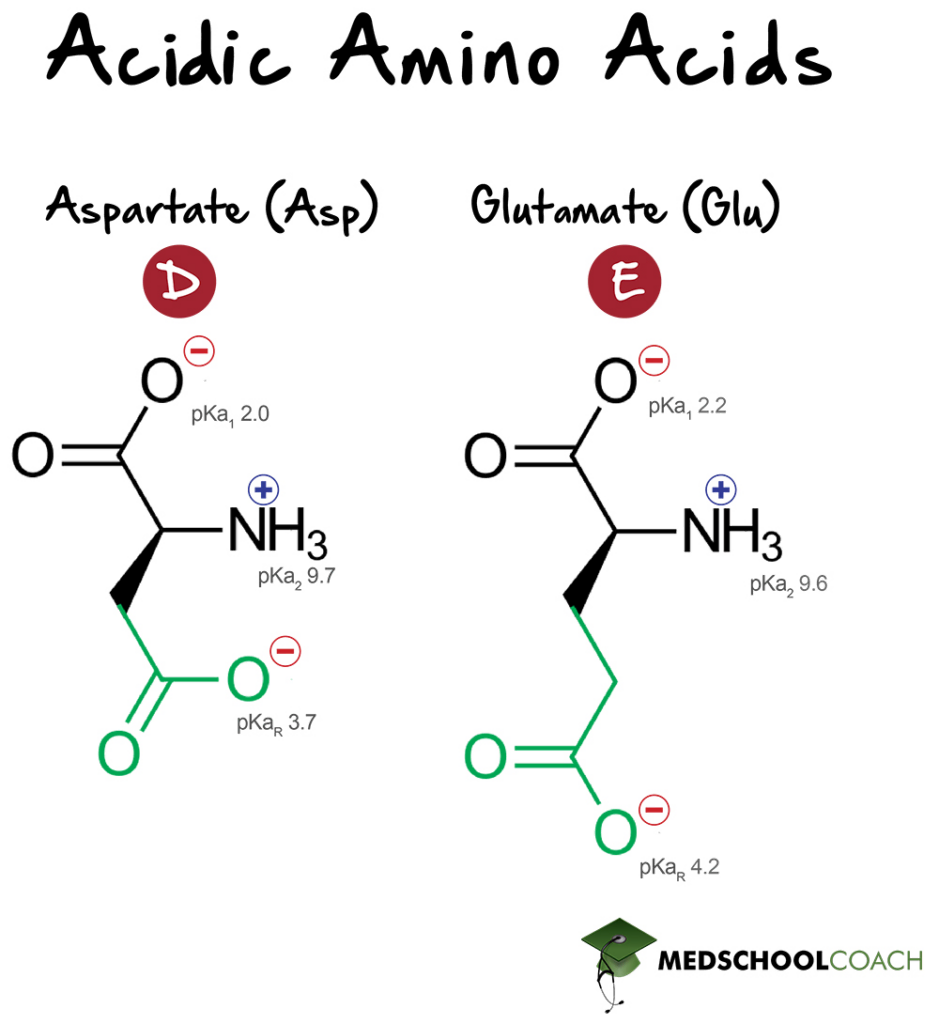
Acidic Amino Acids
Aspartic acid (Asp, D) and glutamic acid (Glu, E) are the only two amino acids with acidic side chains. Aspartic acid’s side chain has a pKa of 3.7 and glutamic acid’s side chain has a pKa of 4.2 When deprotonated, these amino acids are commonly referred to as aspartate and glutamate respectively, in reference to their anionic state. (The fact that the side chains of certain amino acids — including acidic amino acids — can be protonated or deprotonated, and subsequently charged or uncharged, is relevant to the concept of isoelectric point.)
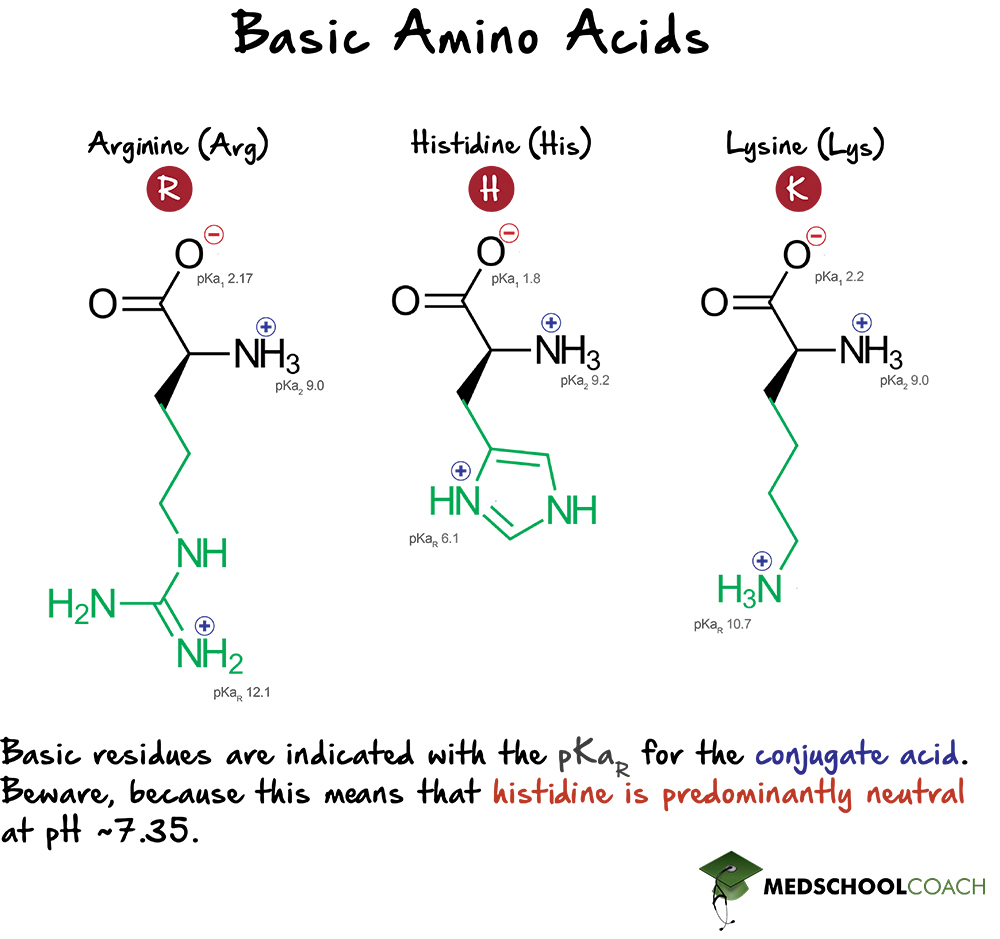
Basic Amino Acids
There are three basic amino acids: histidine, lysine, and arginine. Histidine (His, H) has a side chain pKa 6, lysine (Lys, K) has a side chain pKa of 10.7, and arginine (Arg, R) has a side chain pKa of 12.1. These pKa values, it’s important to note, are those of the conjugate acid. This means that when the surrounding pH is above these pKa’s, these basic side chains will be deprotonated and thus charge-neutral. By contrast, when the pH is below these values, the basic side chains will be protonated and thus positively charged. (Again, this has implications for the concept of isoelectric point.)
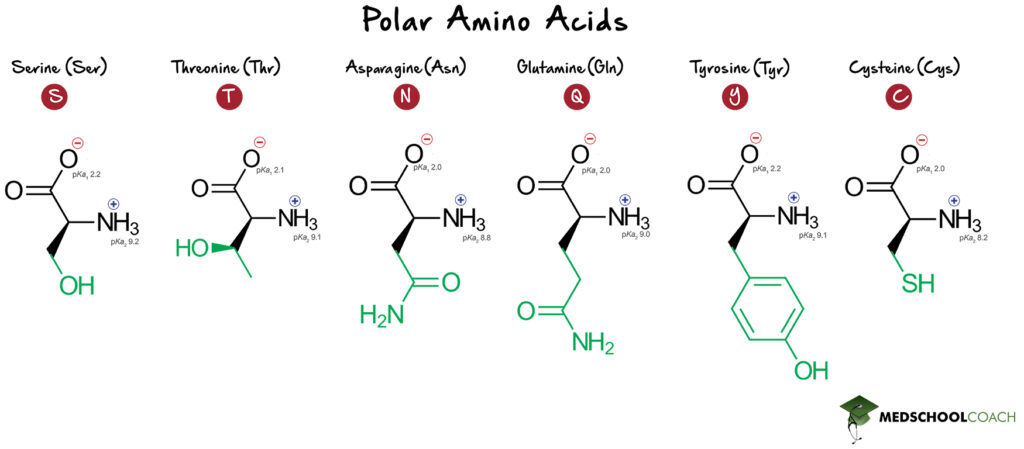
Polar Amino Acids
The polar amino acids that are relevant for the MCAT are serine (Ser, S), threonine (Thr, T), asparagine (Asn, N), glutamine (Gln, Q), tyrosine (Tyr, Y) and cysteine (Cys, C). Notice that all of these amino acids have strong dipole moments in their side chains, but their side chains cannot be charged at any pH.
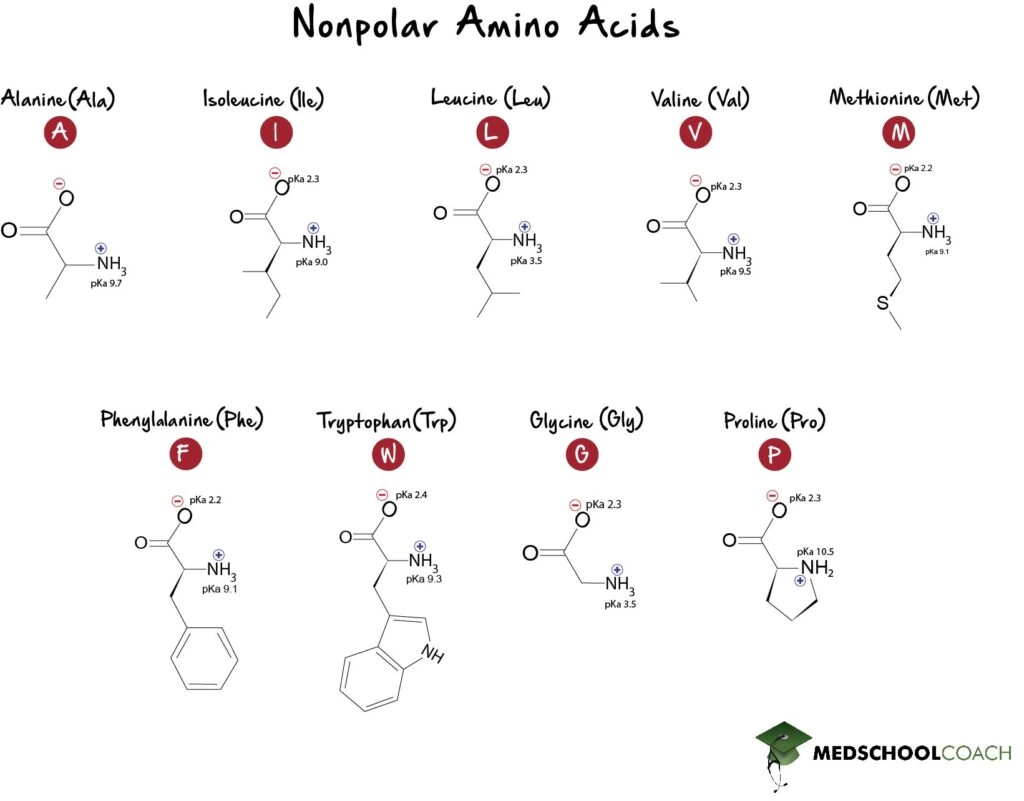
Nonpolar Amino Acids
The nonpolar amino acids are alanine (Ala, A), glycine (Gly, G), isoleucine (Ile, I), leucine (Leu, L), proline (Pro. P), valine (Val, V), phenylalanine (Phe, F), tryptophan (Trp, W), and methionine (M). All these have side chains that are nonpolar and hydrophobic. Because they tend to cluster together, away from the aqueous solvent present in most cellular environments, their hydrophobicity has a large influence on tertiary structure.
This concludes the brief overview of the main way we classify amino acids. The following classifications are additional ways the aforementioned amino acids can also be classified.
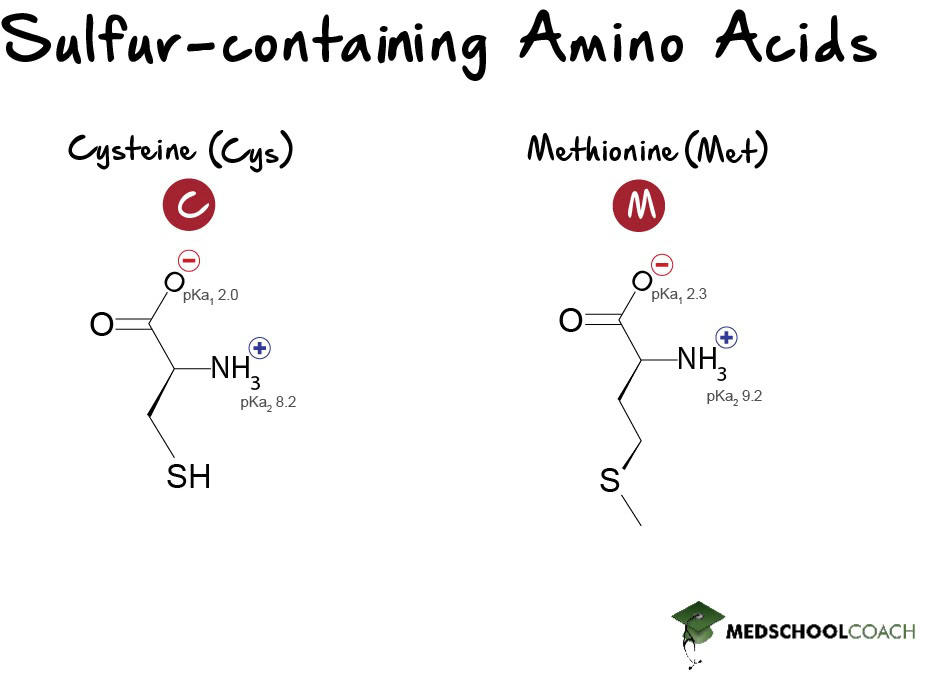
Sulfur-Containing Amino Acids
Cysteine and methionine are the two sulfur-containing amino acids. These amino acids are of particular interest because they form disulfide bonds, which are important for the stability of tertiary and quaternary protein structures.
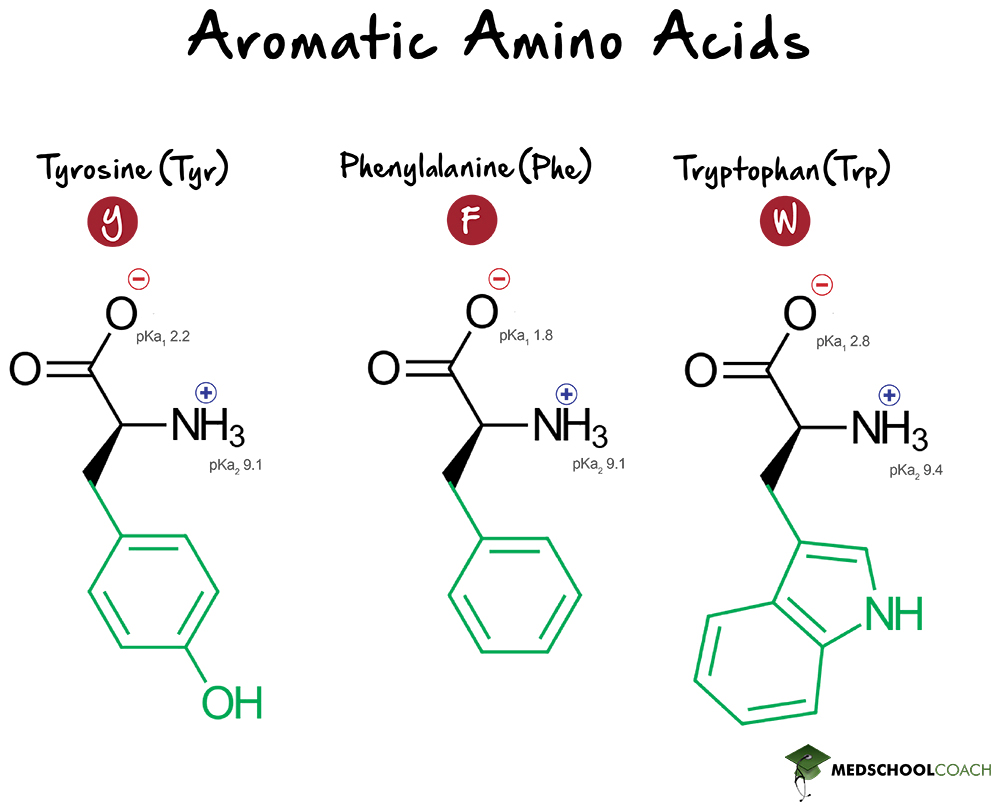
Aromatic Amino Acids
Aromatic amino acids are of interest not only because they are bulky, but also because they serve as precursors for many neurotransmitters. The resonance stabilization present in aromatic rings is also of interest in various experimental techniques. The aromatic amino acids are tyrosine, phenylalanine and tryptophan.
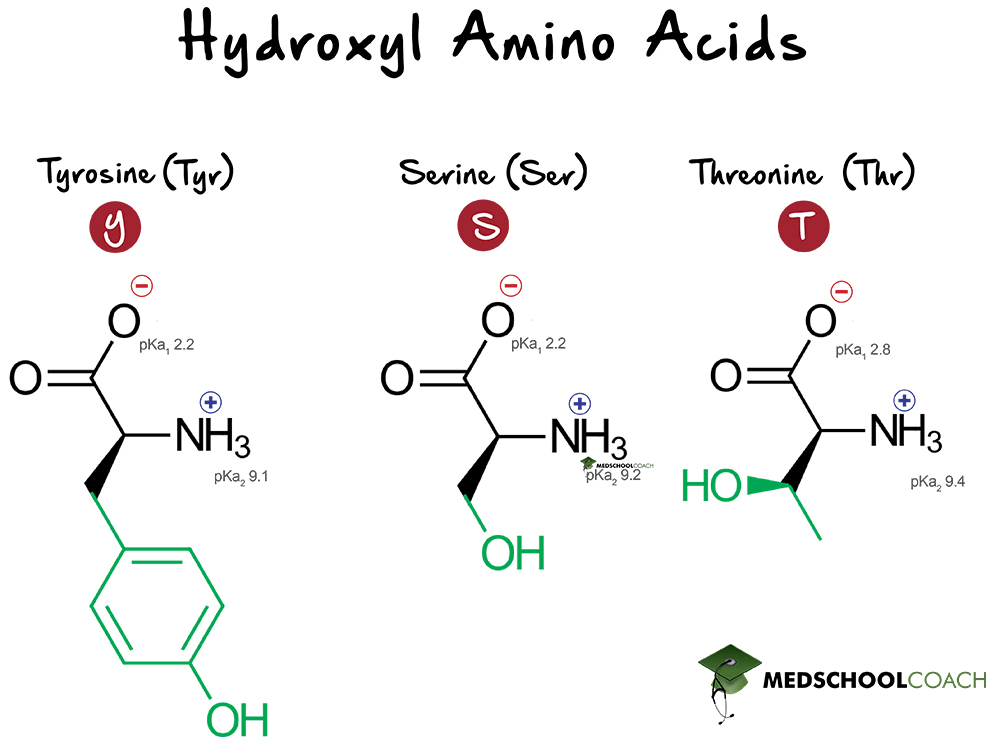
Hydroxylated Amino Acids
Amino acids containing hydroxyl groups in their side chains readily participate in phosphorylation reactions. The three hydroxyl group-containing amino acids are: tyrosine, serine, and threonine.
Amino Acids with Unique Properties
A few amino acids are so structurally distinct that they require a separate discussion. These are glycine, cysteine, and proline. These amino acids are important because the nature of their structure has implications in peptide and protein structure. And because the structure of proteins correlates to their function, it is important to take a closer look at these three amino acids.
Glycine is the only achiral amino acid, as its functional group is a single hydrogen atom. This means that its α-carbon is achiral because it is bound to two identical hydrogen atom substituents. All 19 other amino acids covered in this section are chiral. Additionally, the lack of a bulky R-group allows for significantly easier rotation than any other amino acid. Therefore, glycine is responsible for the flexible regions of peptide chains.
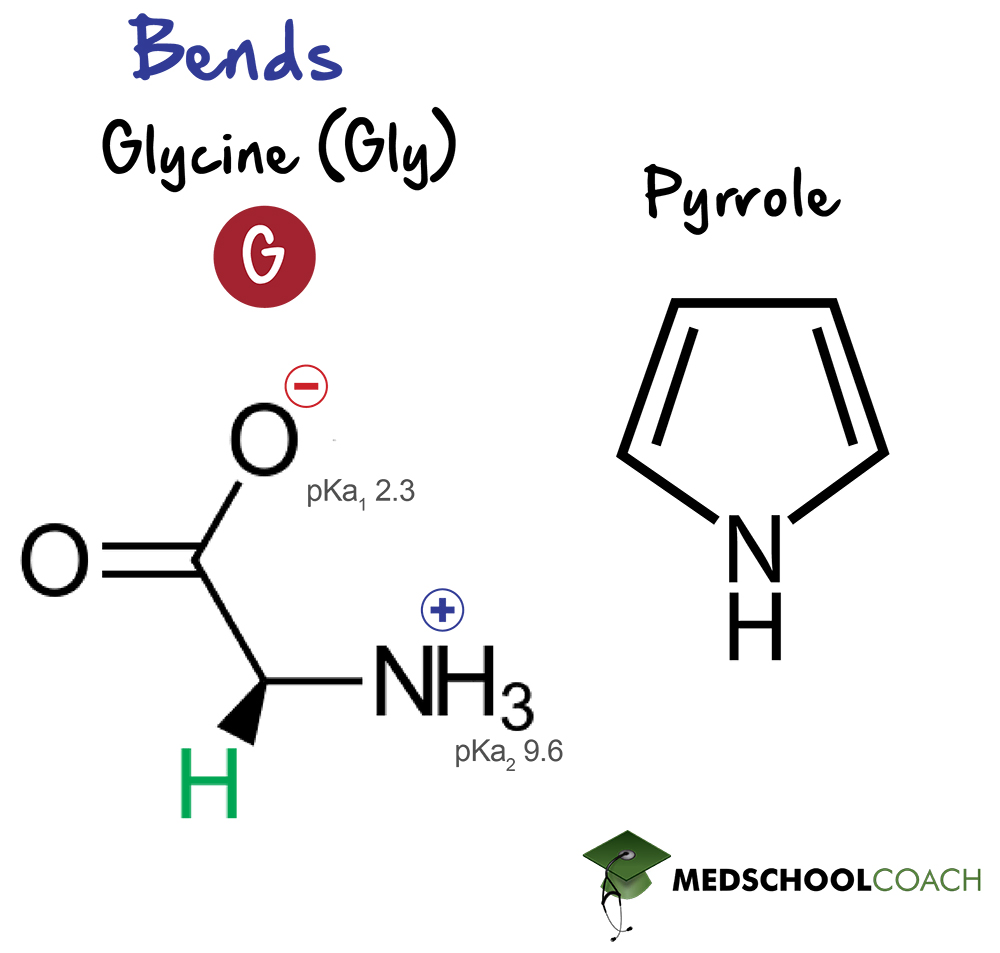
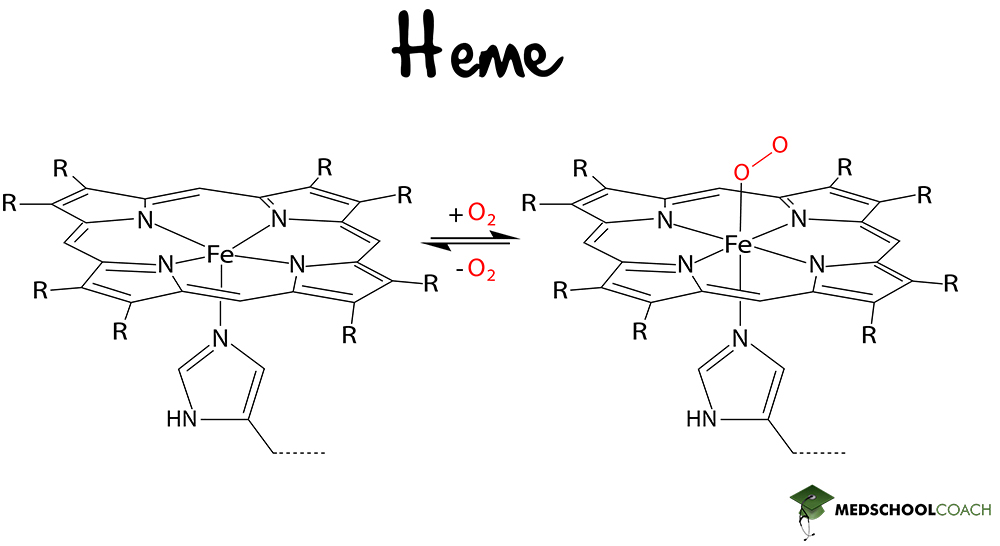
Glycine is also a precursor of pyrrole, an important structural component of porphyrins. Porphyrins are a group of extensively conjugated heterocyclic compounds that readily form coordinate covalent (dative) bonds to transition metal cations, such as ferrous ion (Fe2+). The most notable example of such a compound is heme, the prosthetic group of hemoglobin.
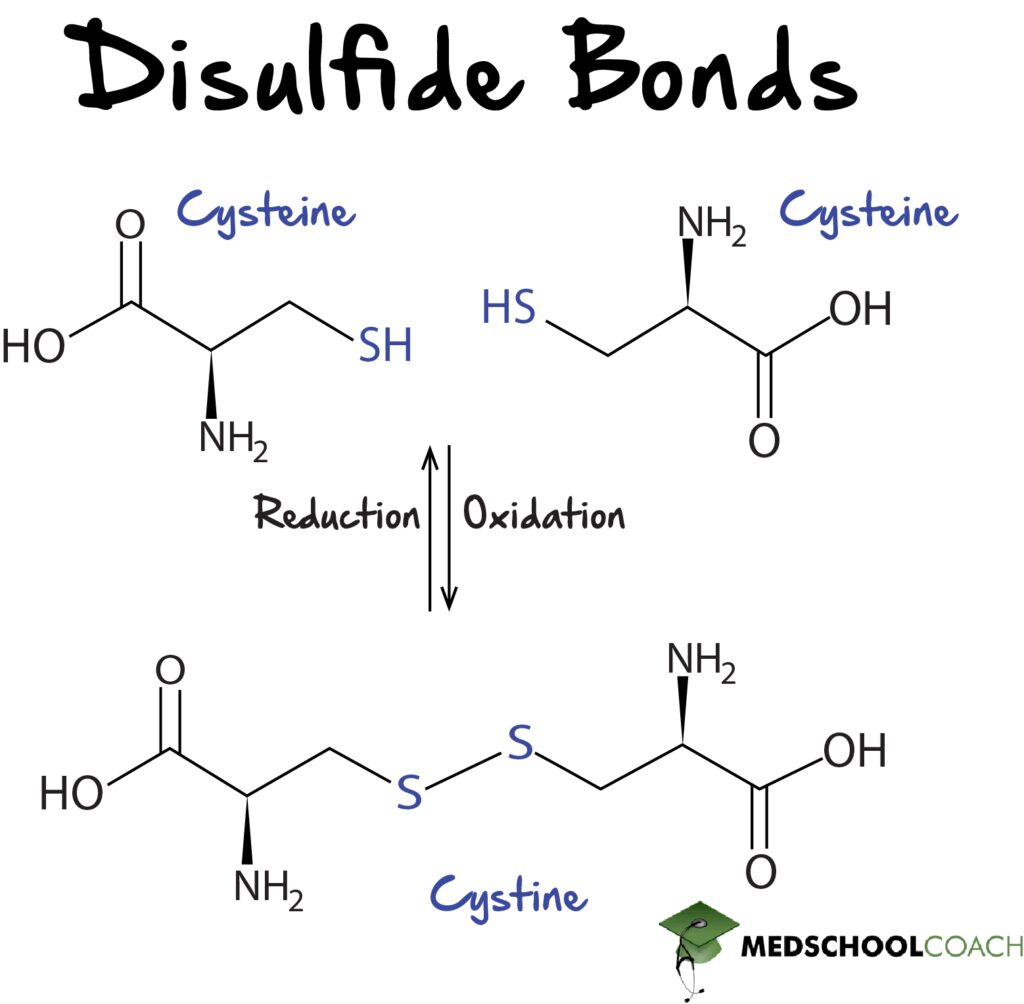
Cysteine is a sulfur-containing amino acid with a thiol group that can form disulfide bonds with other cysteine residues. This allows it to do many things – most importantly, it contributes to the tertiary structure of proteins through the formation of disulfide bonds between non-adjacent amino acids within a protein or peptide.
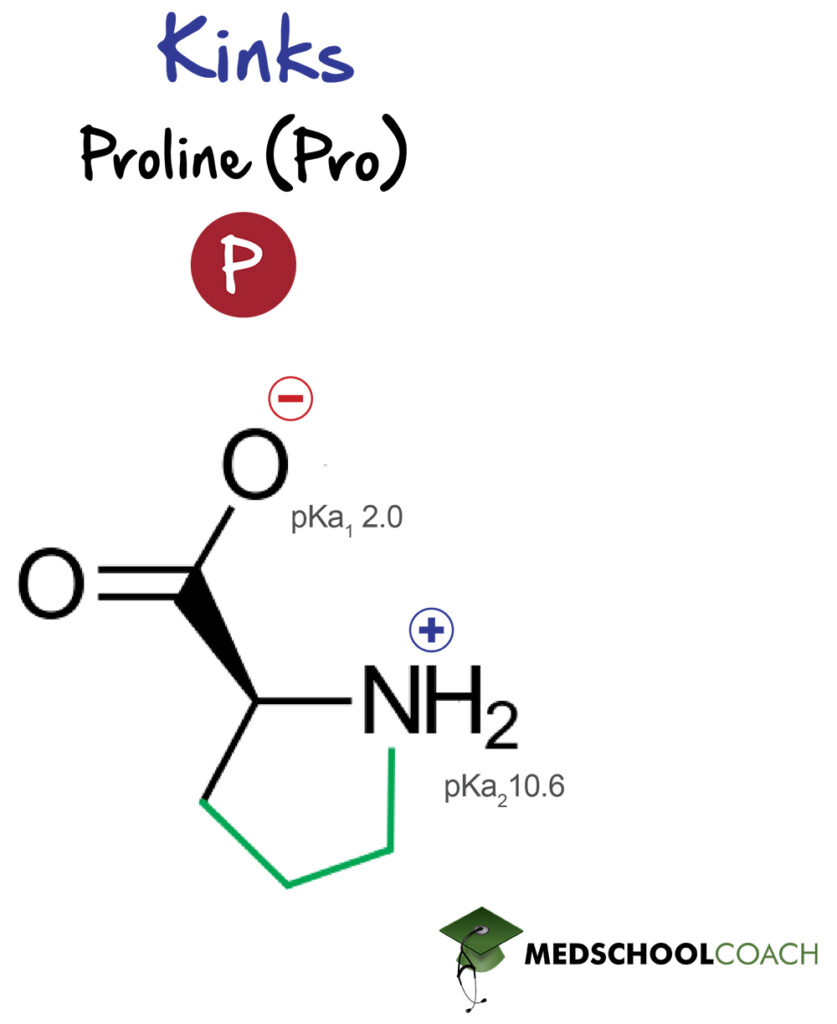
Finally, proline is a fascinating amino acid. It is a cyclized amino acid, and as such is also the only amino acid with a secondary amine group (or tertiary when incorporated into a peptide bond). This disrupts common secondary structure motifs and has a significant effect on the secondary structure of proteins by introducing a forced change in angle, a kink, due to its far greater rigidity than most other amino acids.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.