Amino Acid Configuration and Structure
MCAT Biology Chapter 1 - Section 1.1 - Amino Acids - Amino Acid Configuration and Structure
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Amino Acids
- »
- Amino Acid Configuration and Structure – MCAT Biochemistry
Sample MCAT Question - Amino Acid Configuration and Structure
Which of the following statements are always true?
I. Amino acids are amphoteric.
II. D-amino acids rotate light clockwise.
III. L-amino acids rotate light clockwise.
a) I only
b) I and II only
c) II and III only
d) I, II, and III
A is correct. I only.
A is correct. Amino acids have both an acidic carboxyl group (-COOH) and a basic amino group (-NH2). Therefore, amino acids are amphoteric, meaning they can react as both an acid and a base. The D/L configuration terminology used to describe the stereochemistry of amino acids is not related to the d/l configurations for optical activity. Therefore, D-amino acids do not necessarily rotate light clockwise and L-amino acids do not necessarily rotate light clockwise. Out of the three options, only choice I is true.
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Amino Acid Configuration and Structure
The topic of amino acids, particularly the 20 proteinogenic α-amino acids of eukaryotes, is a very high yield MCAT topic. For this reason, the study of their properties and structure should not be taken lightly during your content review time. This post will cover the basic structure of amino acids, as well as amino acid configuration at the α-carbon. As we’ll discuss, amino acids can be described in terms of their absolute configuration (R and S notation) or their relative configuration (L and D notation).
Amino Acid Structure (Basic)
The backbone, or basic structure of every amino acid is shown in Figure 1. This backbone consists of a chiral α-carbon bonded to four different groups. These groups are the carboxyl group, the amino group, a hydrogen, and 1 out of 20 possible side chains. Note from this basic structure that every amino acid has at least two ionizable groups; each has at least one acidic group (carboxyl group) and one basic group (amino acid) and possibly another ionizable group in its side chain. These ionizable groups have various pKa values and can adopt various protonation states. Additionally, amino acids are classified based on the properties of their side chains — amino acids with ionizable side chains are either acidic or basic.
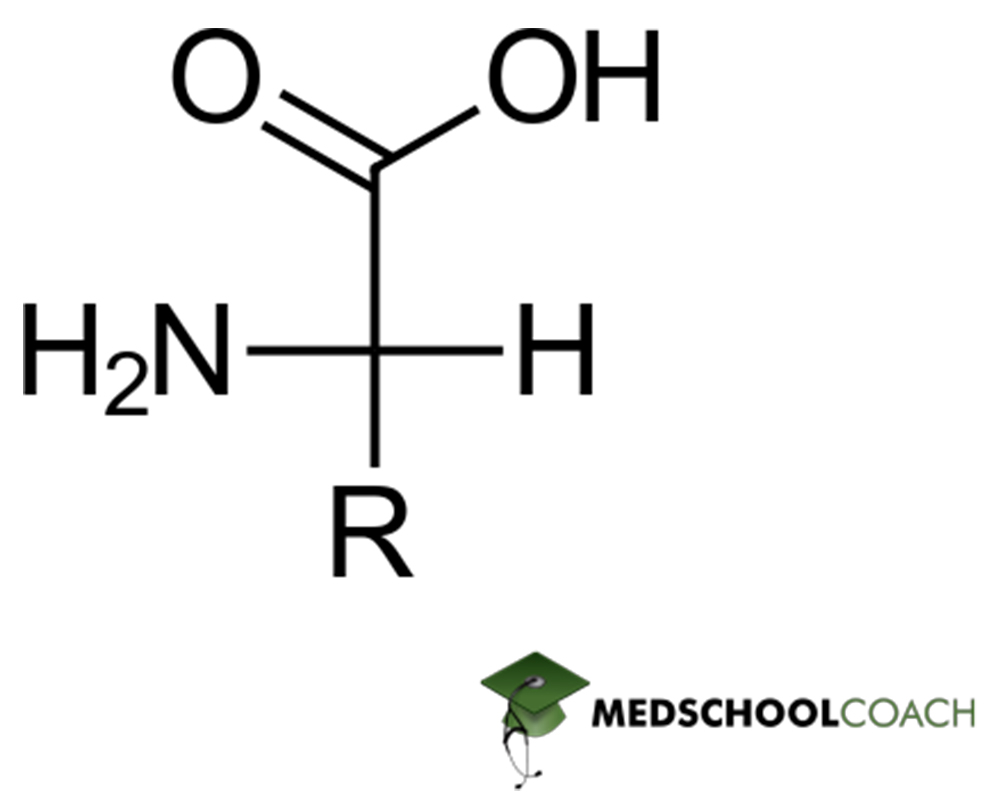
Now, the fact that the α-carbon is bonded to four groups should make you suspect that most amino acids may be chiral. This is true. And as you may also be able to extrapolate, if the R-group is simply a hydrogen, then the molecule loses its chirality, because now two identical substituents are bound to the α-carbon. This is the case with glycine. While glycine clearly lacks a chiral center, the remaining 19 of the 20 amino acids are all chiral.
Since we now know that most amino acids are chiral, and therefore can have enantiomers, we need to discuss the different ways we can classify a molecule with a single stereocenter. Enantiomers can be classified in three ways:
- – Absolute configuration
- – Relative configuration
- – Optical properties
Absolute Configuration of Amino Acids
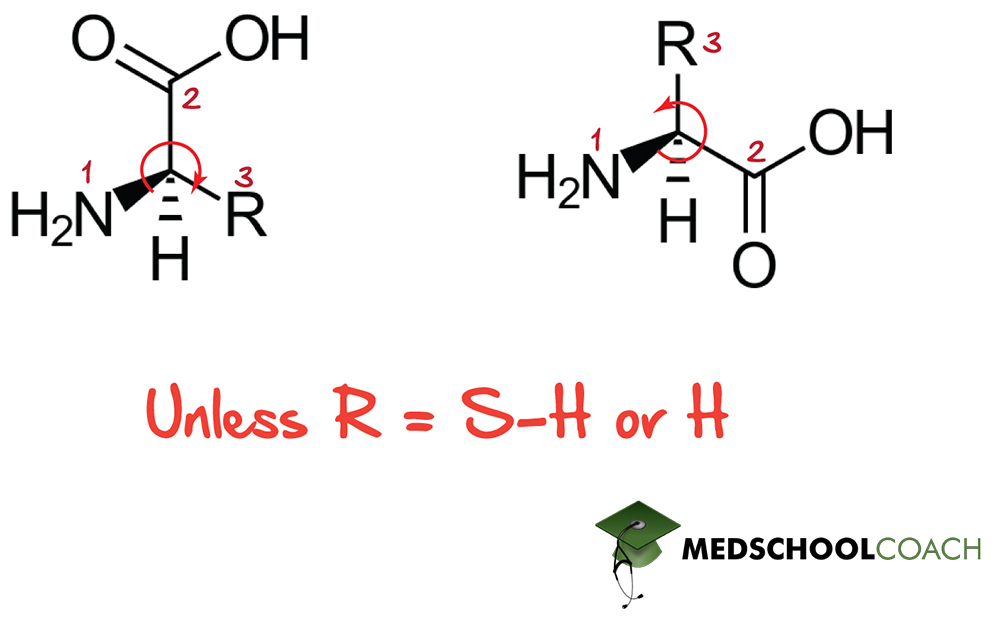
Absolute configurations, as those described in figure 2, are those you are likely most familiar with. These are those that use Cahn-Ingold-Prelog priority assignment for substituents to assign each group a ranked priority and then determine a handed-ness (R or S) on the basis of the ranked order for substituents 1-3, provided that the lowest-ranked substituent has been placed in the back. Absolute configurations have the advantage that they are unambiguous, clear, consistent, and recognizable in any sort of projection. They can however be somewhat clumsy to figure out, and for historical reasons, are not always the go-to method of identifying amino acids or monosaccharides. Most α-amino acids are in the S configuration – however, since sulfur has a high CIP priority, cysteine proves to be an exception and appears in the R configuration.
Relative Configuration of Amino Acids
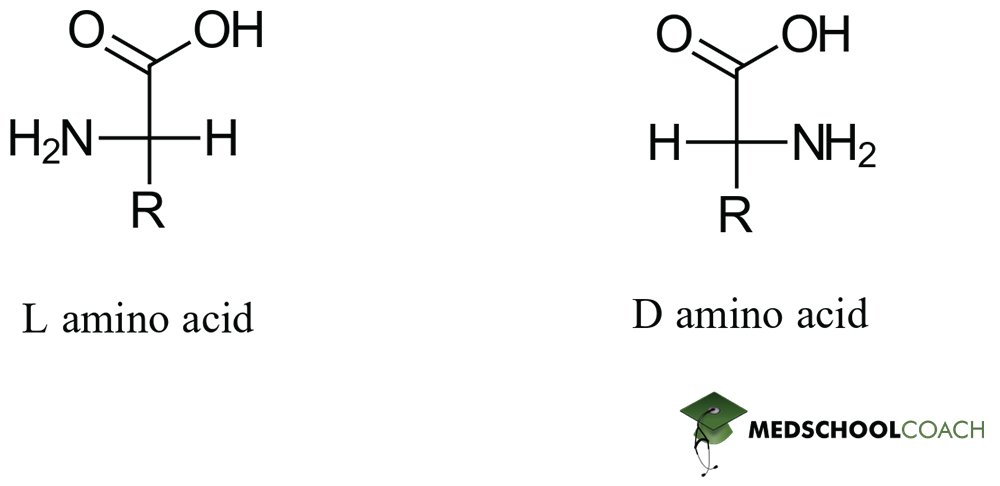
Relative configurations are those which determine configurations of small chiral molecules by their homology to glyceraldehyde, something called the Fischer convention. This is a very common way of naming α-amino acids (and sugars), but it can feel somewhat unusual and unsystematic to students. Note that all amino acids are synthesized in the L configuration, although on extremely rare occasions enzymes called racemases may convert some amino acids to the D configuration. Also take heed, there is no systematic relation between absolute and relative configuration – a vast majority of L amino acids are also S, but there is no guarantee that this is the case. Always verify!
Optical Activity of Amino Acids
As a final method of classification, we have optical activity, denoted with lowercase d and l (or + and –), which stands for dextrorotatory versus levorotatory for clockwise and counterclockwise rotation of plane polarized light. This property is determined experimentally and cannot be predicted on hand of the structure alone.
If you’re looking for more info about amino acids, our other MCAT content posts can teach you how:
– to classify amino acids based on the properties of their side chains (acidic, basic, polar, nonpolar, etc.)
– to calculate isoelectric point based on the pKa values of ionizable groups in amino acids (α-amino, α-carboxyl, side chain)
– peptide bonds join together amino acids to form peptides and proteins
– amino acids are synthesized via Strecker synthesis or Gabriel synthesis
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.