Peptide Bonds
MCAT Biochemistry Chapter 1 - Section 2.1 - Amino Acids - Peptide Bonds
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Amino Acids
- »
- Peptide Bonds – MCAT Biochemistry
Sample MCAT Question - Peptide Bonds
Which of the following is true about peptide bonds?
I. No free rotation
II. Require enzymes to break these bonds
III. Breaking these bonds is exergonic
a) I and II only
b) I and III only
c) II and III only
d) I, II, and III
D is correct. I, II, and III.
D is correct. Peptide bonds have partial double bond character due to resonance. Therefore, they are less flexible than other single bonds and have no free rotation (I is correct). They are also stronger due to this partial double bond characteristic. Therefore, even though breaking peptide bonds is an exergonic process (III is correct), it stills has a high activation energy that require the use of enzymes (II is correct). Answer choices A, B, and C are incorrect because they do not include all of the correct choices.
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Intro to Peptide Bonds
Amino acids are the monomer building blocks of peptides and proteins. In other posts, we discussed the structure and configuration of amino acids, their classification (as polar, nonpolar, etc.), and their protonation states and isoelectric point. In this post, however, we’ll discuss how amino acids link together via peptide bonds and how these bonds can be broken via hydrolysis. We’ll also discuss the special resonance character of peptide bonds, which gives them extra strength.
Peptide Bond Formation and Hydrolysis
Peptide bond formation is endergonic and has a high activation energy, meaning there is both a thermodynamic and kinetic barrier to the formation of peptide bonds. The thermodynamic barrier can be overcome by coupling peptide bond formation to exergonic reactions – in biological systems, this is done almost exclusively through coupling to ATP hydrolysis. The kinetic barrier, in turn, can be significantly lowered with the use of biological catalysts, more commonly called enzymes. Conversely, since thermodynamic favorability (Gibb’s free energy) is a state function, if the formation of peptide bonds is endergonic, this suggests that the hydrolysis of peptide bonds must be exergonic. Meaning that proteins spontaneously break down in aqueous solution. The hydrolysis of peptide bonds is spontaneous in vivo, but often extremely slow due to a high activation barrier for these hydrolysis reactions. Enzymes that catalyze peptide bond hydrolysis reactions exist as well and are called proteases.
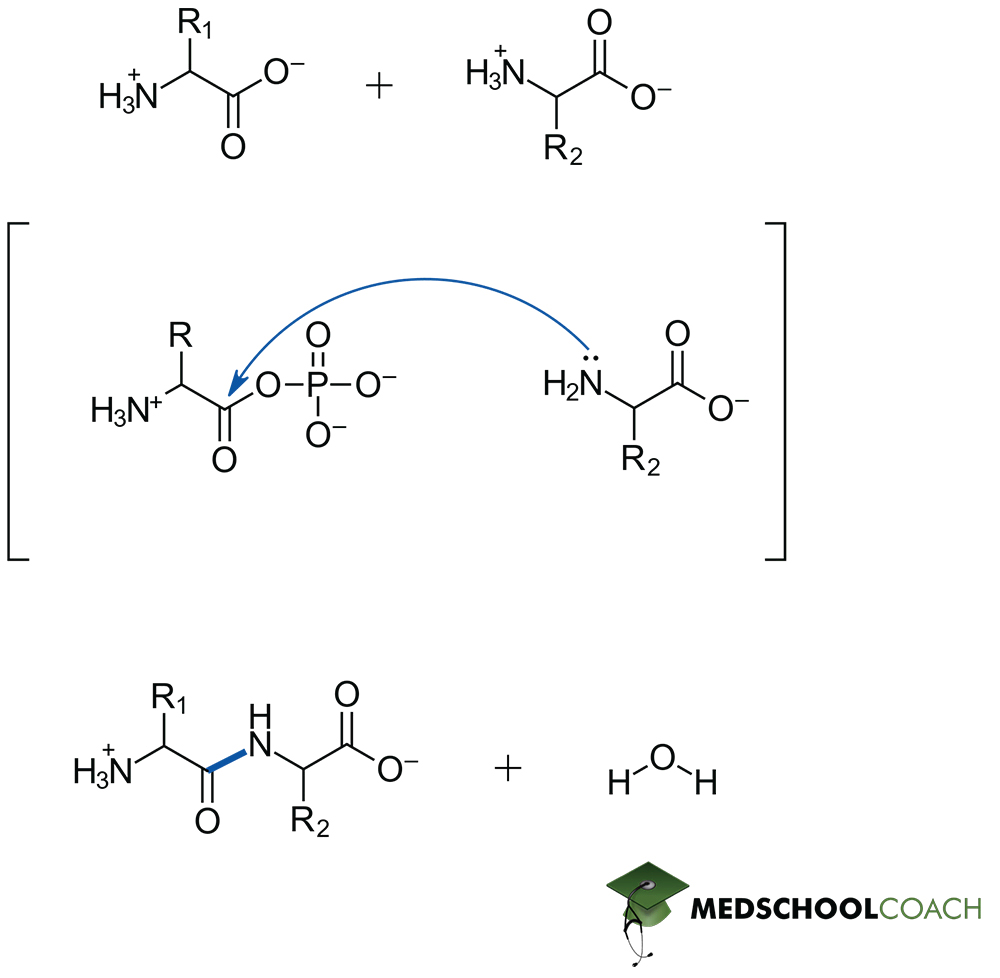
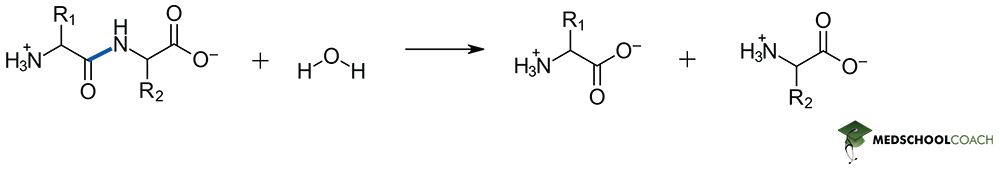
Peptide bond formation is a condensation reaction, meaning that it will result in the formation of water as a separate product. Figure 1 describes key steps in this process. Notice that the lone pair of an amine acts as the nucleophile, and therefore the resonance stabilized carboxylate is not a viable electrophile. In living systems, formation of a phosphoester from ATP allows for the generation of a viable electrophilic site on the carbonyl carbon and therefore the reaction can proceed. Peptide bond hydrolysis on the other hand is a dehydration reaction – and while the in vivo mechanism of catalyzed hydrolysis reactions may also appear on the MCAT, such mechanistic details can be expected to be given from a passage directly. Most importantly this is a hydrolysis reaction, so water is required as a reagent.
Identifying Peptide Bonds
Now that we know how peptide bonds are formed, we should have an easy time identifying them. Refer to the dipeptides in figure 3 whose peptide bonds are indicated in blue. Notice that in a simple dipeptide, one amino acid presents an exposed amine group (the N-terminus) and another presents an exposed carboxylate (the C-terminus). For a simple dipeptide, this already means that the order of bond formation matters for the resulting structure – as N-Ala-Tyr-C is not equivalent to N-Tyr-Ala-C, as demonstrated in Figure 3. In one instance, alanine has a free N-terminus and tyrosine has a free C-terminus, and in the other instance this relation is reversed.
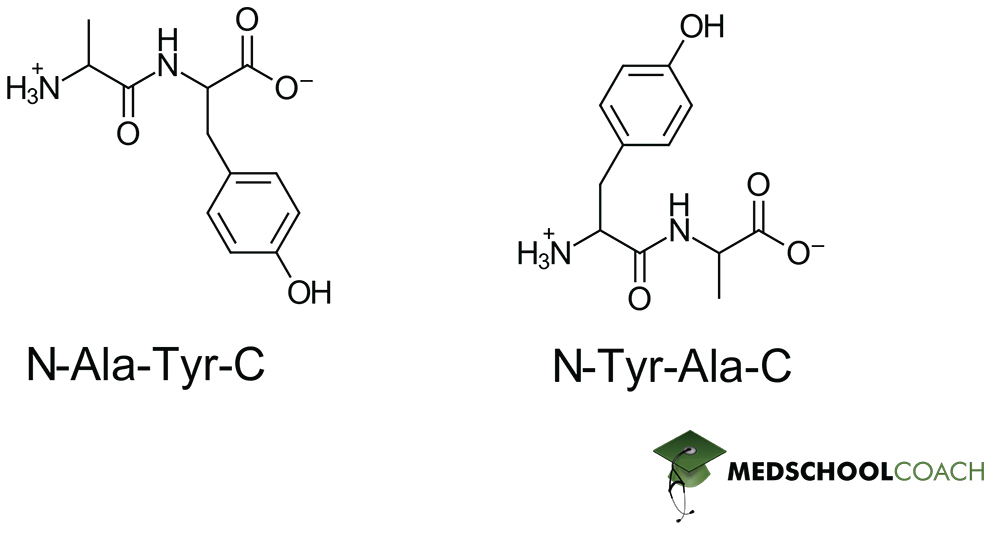
This is an interesting property of peptide bonds, as it implies that the order of polymerization matters for the final structure. And more concretely, for taking the MCAT this means that you want to be paying close attention to any indication of where the N and C termini lie – because skipping this step can lead to you fundamentally misunderstanding the structure of a given peptide!
Resonance in Peptide Bonds
What we’ve so far been referring to as simply the peptide bond, is in actuality the resonance hybrid of two individual Lewis structures. While conventionally depicted as the left-hand structure in Figure 4, a peptide bond is really something more akin to an intermediate between the left- and righthand structures. The lone pair from the nitrogen atom can participate in resonance, thereby forming the structure on the right. Keep in mind however, that while some common resonance hybrid are the result of two equal contributors (such as the resonance hybrid of the carboxylate moiety), in this instance this is not the case. The left-hand structure contributes far more significantly to the overall structure of the peptide bond than the one on the right.
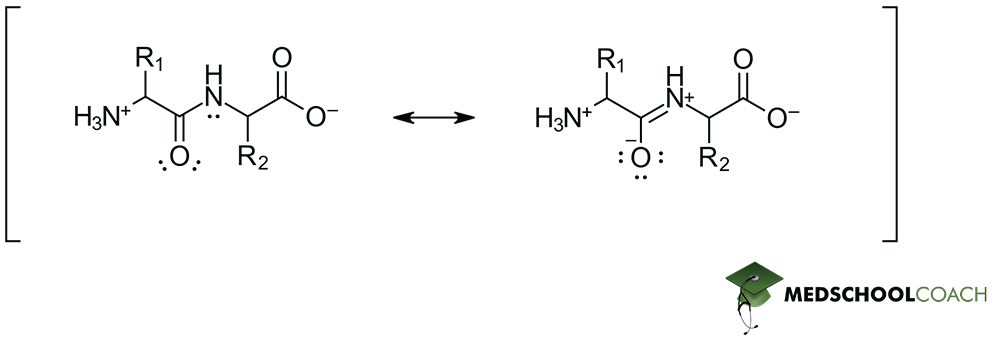
This has two important consequences:
First, it implies that peptide bonds are strong – stronger than their single-bond depiction might suggest, as the resonance hybrid lowers the energy of the bond and makes it a more thermodynamically favorable state to remain in. This is important because the function of biological systems relies heavily on the relative stability of proteins.
Second, the fact that the lone pair of nitrogen is participating in resonance means the effective hybridization of the nitrogen atom must be sp2, which in turn results in a planar geometry at that nitrogen. Combined with the sp2 hybridized carbonyl carbon, this means that the entire peptide bond forms a single planar arrangement that does not rotate relative to itself – leaving only the bonds around the α-carbon to freely rotate within a polypeptide.
Ultimately, these two properties are major reasons that amino acids are stable over time and do not hydrolyze easily without the help of enzymes.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.