Gel Electrophoresis, PAGE, SDS- PAGE
MCAT Biochemistry Chapter 2 - Section 2.1 - Peptides and Proteins - Gel Electrophoresis (PAGE, SDS- PAGE)
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Gel Electrophoresis, PAGE, SDS- PAGE – MCAT Biochemistry
Sample MCAT Question - Gel Electrophoresis, PAGE, SDS- PAGE
Which technique used in gel electrophoresis can separate proteins by size only?
a) Reducing SDS-PAGE and SDS-PAGE
b) Native PAGE only
c) SDS-PAGE only
d) Native PAGE and SDS-PAGE
C is correct. SDS-PAGE only.
There are different gels used in gel electrophoresis that have unique properties. Sodium dodecyl sulfate (SDS)-PAGE is a gel used to separate proteins by size only, and not charge. SDS is a detergent that will bind to proteins and denature them. Furthermore, when it binds to the molecule, the SDS detergent surrounds it with a negative charge. The benefit of surrounding the protein with the same charge is that during gel electrophoresis, all the proteins will run in the same direction, allowing molecular separation by size. Answer choice B and D are incorrect because native PAGE separates proteins in their native conformation by size and charge. Answer choice D is incorrect because reducing SDS-PAGE is SDS-PAGE under reducing conditions, which can break disulfide bonds, and is useful for detecting the presence of quaternary structures held together by these bonds.
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Gel Electrophoresis
Gel electrophoresis is a widespread laboratory technique that is used to separate molecules by size and charge. The gel that is used in this technique is made of a polymer that contains pores of various sizes. Conventional polymers include agar as well as polyacrylamide. The purpose of having pores of various sizes in the gel is for molecules to be able to move through the gel. During gel electrophoresis, larger molecules move through the pores more slowly than smaller molecules, and this process separates the molecules by their size.
In order to separate molecules by charge, gel electrophoresis uses an electrolytic cell. Figure 1 shows the electrolytic cell, which has a positive end, or anode, and a negative end, or cathode. The gel is placed inside the electrophoretic chamber, and the sample is introduced into the wells on one end of the gel. The machine is then turned on, and the molecules will migrate through the gel according to their size and charge. In this way, the positively charged molecules will move toward the cathode, and the negatively charged molecules will move towards the anode.
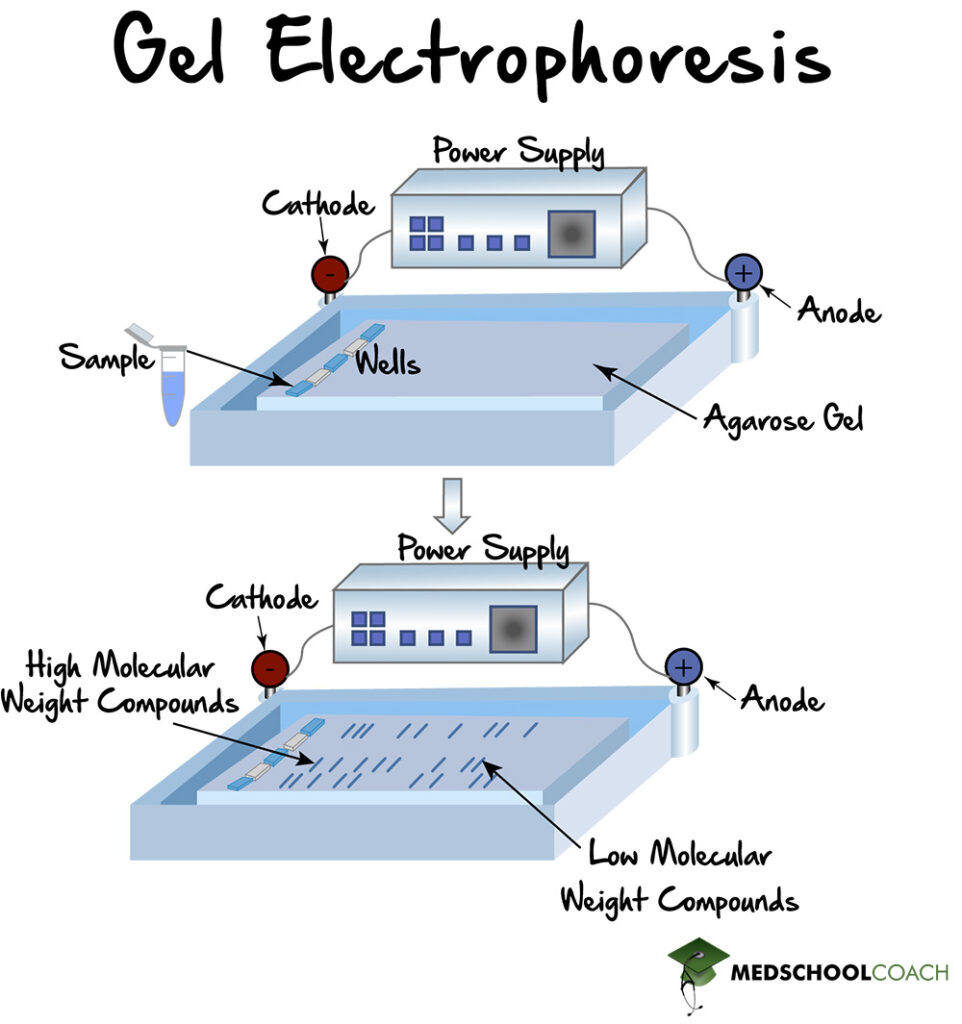
In certain situations, such as with DNA, all of the molecules have a similar charge (DNA has a negative charge). In these cases, all the molecules will move towards one side, so separation is achieved solely by the pores in the gel. The smaller molecules will move through the gel faster, and the larger molecules will move through the gel more slowly.
Different Types of PAGE
There are three different types of polyacrylamide gel electrophoresis (PAGE) that are important to know for the MCAT. There is native PAGE, SDS-PAGE, and reducing SDS-PAGE.
Native PAGE
Native PAGE gel electrophoresis is electrophoresis under native conditions. In other words, proteins are separated by size and charge in their native conformations without any intermediate steps, such as denaturation. Furthermore, proteins in their native conformations exist in different shapes and forms that affect the way the protein will migrate through the pores in the gel. Some shapes and forms will migrate through the pores more easily than others. Moreover, unlike DNA molecules that consist entirely of negative charges, proteins can have both positive and negative charges. In native PAGE gel electrophoresis, positively charged proteins will move toward the negatively charged cathode, whereas negatively charged proteins move towards the positively charged anode. Native PAGE is generally the least used gel because there are so many confounding factors that can affect separation.
SDS-PAGE
SDS-PAGE is gel electrophoresis using a chemical detergent called sodium dodecyl sulfate (SDS) (Figure 2). As a detergent, sodium dodecyl sulfate is an amphipathic molecule, meaning that one side of the molecule is polar and the other side is non-polar, allowing SDS to interact with both polar and non-polar parts of proteins.
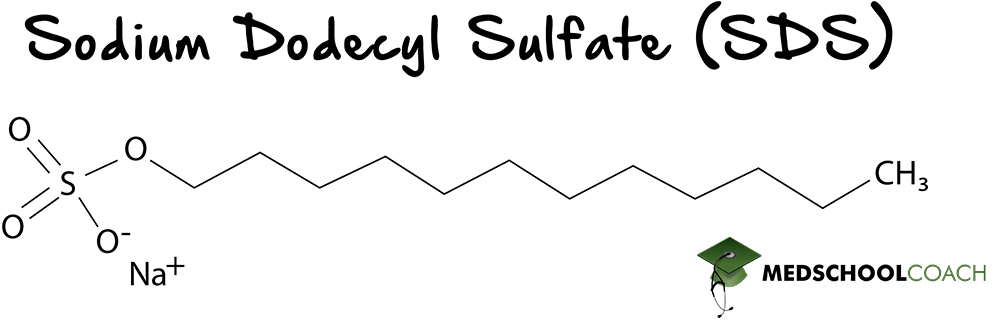
Initially, the protein is folded (has a secondary, tertiary, and possibly quaternary structure) and has positive and negative charges. The overall charge of a protein depends on the balance of those charges. As a detergent, SDS will denature proteins, resulting in straight chains of amino acids (Figure 3). When this happens, the proteins will become surrounded by SDS molecules, giving the proteins an overall negative charge. If the gel is run at this point, all the proteins will migrate in the same direction towards the positively charged anode. Also, since all of the proteins have the same charge, they will only be separated by their size. In other words, SDS-PAGE eliminates the influence of charge during gel electrophoresis. The protein is solely separated based on the length of the polypeptide chain.
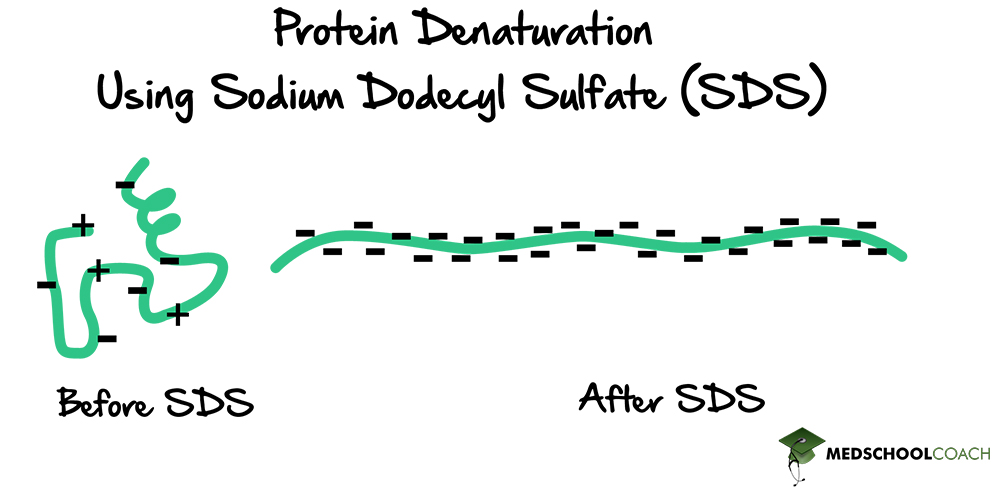
Reducing SDS-PAGE
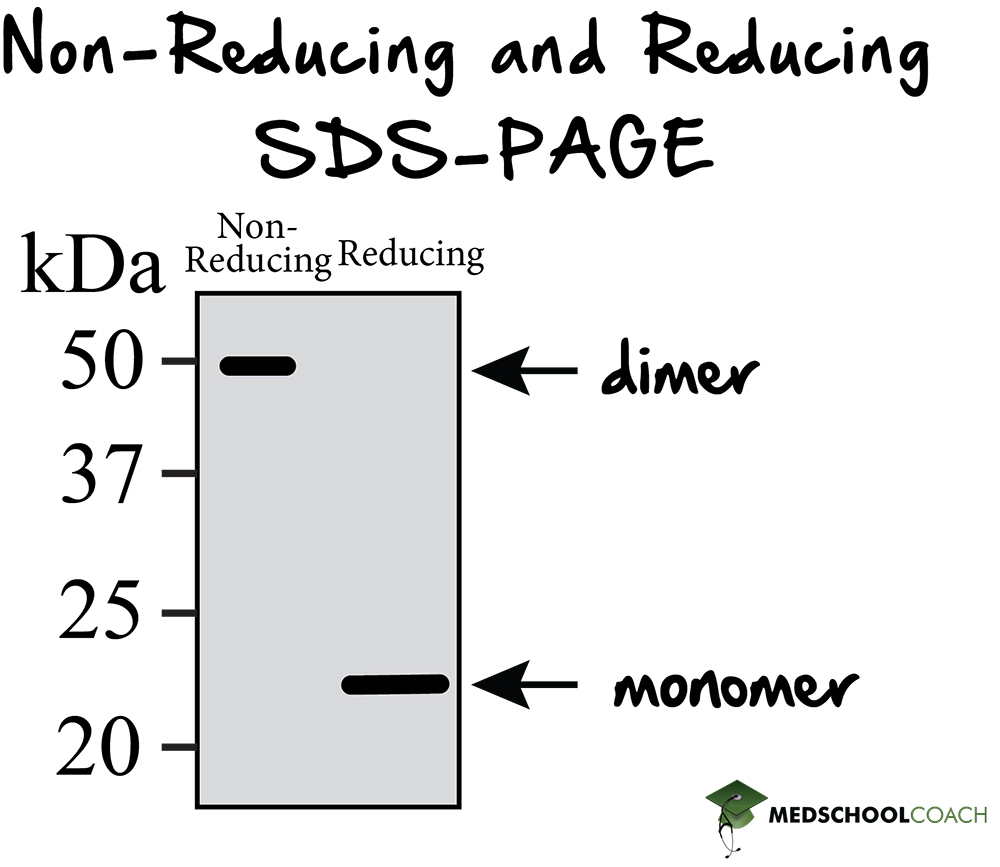
Reducing SDS-PAGE is SDS-PAGE with the addition of a reducing agent such as beta-mercaptoethanol. A reducing agent can break disulfide bonds, and for a majority of proteins, this will not make much difference in terms of how the protein presents on the gel. However, for those proteins with quaternary structure, reducing SDS-PAGE is useful in that it breaks the protein down into its subunits by disrupting the covalent interactions between them. Figure 4 shows an example of running a reducing SDS-PAGE gel. There are two columns, one for non-reducing conditions, or regular SDS-PAGE, and another column for reducing conditions, or reducing SDS-PAGE. In reducing conditions, the higher molecular weight structure is missing. The molecular weight of these structures is a result of dimers, which are a form of quaternary structures. In the reducing conditions column, the dimers have been separated. This result indicates that the dimers in this protein are held together by disulfide bonds, which gives the investigator a better picture of the initial protein structure.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.