Find out everything you need to know about Enzyme Inhibition of Michaelis-Menten Kinetics, this includes the differences between competitive, uncompetitive, non-competitive, and mixed inhibition.
Enzyme Inhibition
MCAT Biochemistry - Chapter 3 - Section 2.2 - Enzymes - Enzyme Inhibition
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Enzyme Inhibition – MCAT Biochemistry
Sample MCAT Biochemistry Question
In competitive enzyme inhibition, which one of the following is true?
a) KM decreases because the binding of a competitive inhibitor decreases the enzyme-substrate affinity
b) The inhibitor binds to an allosteric site, not an active site
c) KM increases and more substrate is required to reach 1/2Vmax
d) Vmax increases because increasing the inhibitor concentration increases the maximum rate of reaction
C is correct. KM increases and more substrate is required to reach 1/2Vmax
By binding to an enzyme, an enzyme inhibitor can decrease its activity. In competitive enzyme inhibition, substrate and inhibitor molecules compete to bind at the enzyme’s active site. This decreases the affinity of the substrate for the enzyme, increasing KM. This also means that it will take a greater substrate concentration to reach 1/2Vmax. Answer choice A is incorrect because the Km does not decrease with competitive inhibition. Answer choice B is incorrect because competitive inhibitors binds to the active site, not allosteric sites. Answer choice D is incorrect because inhibitors, by definition, do not increase the Vmax of a reaction.
Enzyme Inhibition Intro
Enzymes are biological catalysts, most commonly consisting of a protein or group of proteins, that increase the rate of chemical reactions. Enzymes are reaction specific, meaning that they usually only catalyze one reaction and no others. They can also be inhibited, meaning their ability to do their job is hampered. In this post, we’ll discuss the different types of enzyme inhibition and how they work.
Recall that the fundamental reaction in Michaelis Menten kinetics is:
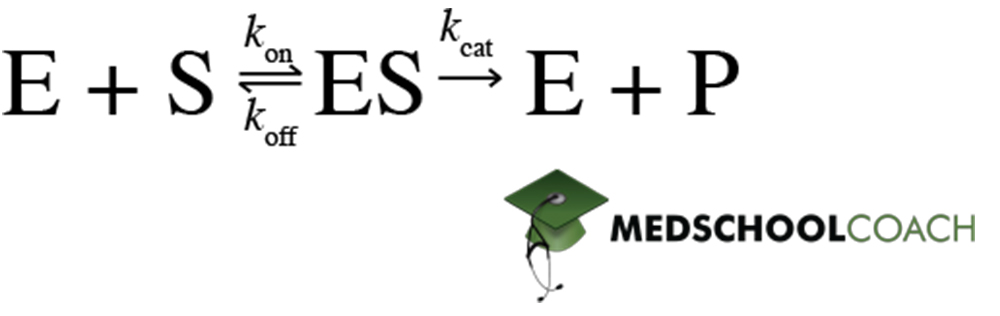
Different enzyme inhibitors interfere at different points of the overall process illustrated by the equation above. They will therefore affect the Michealis-Menten saturation curve and equation variables in different ways: some inhibitors will affect KM but not Vmax, others will affect Vmax but not KM, and others still will affect both variables. Read on to find out which types of inhibition affect which variables.
Competitive Inhibition
In competitive inhibition, the inhibitor binds directly to the enzyme to form an enzyme-inhibitor complex. This enzyme-inhibitor complex cannot react, so when it forms, the enzyme will be unable to produce the product. In this way, the reaction is stopped from moving forward. Moreover, it is crucial to understand where an enzyme will bind to an inhibitor. In competitive inhibition, the inhibitor is competing directly with the substrate to bind to the enzyme. In normal circumstances, the substrate binds to the enzyme’s active site. Similarly, competitive inhibitors also bind to the enzyme’s active site.
Competitive inhibition affects KM. Recall that the KM is inversely related to the affinity of the enzyme for the substrate. In this way, when an inhibitor is added, it makes it harder for the enzyme to bind to its substrate. Although the enzyme wants to bind to the substrate, the inhibitor gets in the way. In this way, in the presence of competitive inhibition, the affinity of the enzyme for the substrate decreases. Similarly, the value of KM increases.
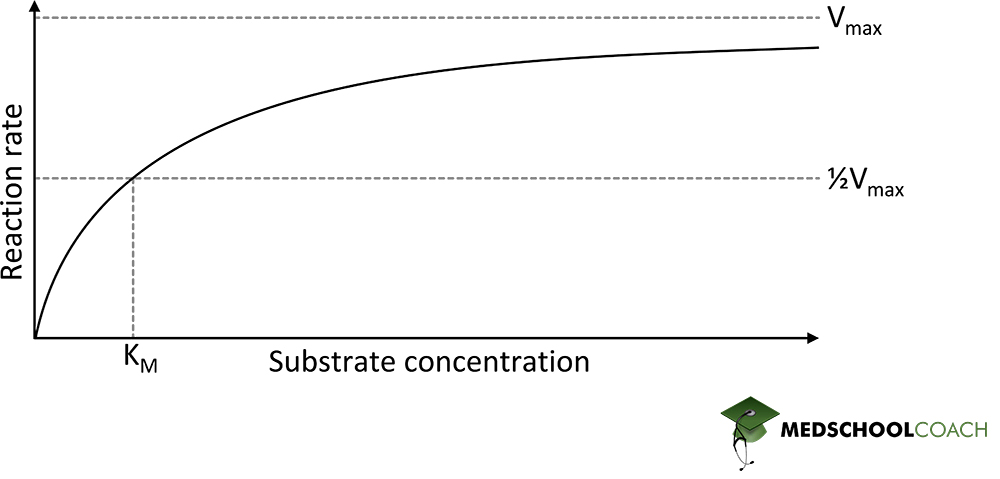
In terms of the maximum velocity of the reaction, or Vmax, recall the Michaelis Menten saturation curve shown in Figure 1. The relevant questions is whether the inhibitor is going to affect the maximum reaction velocity? In terms of competitive inhibition, the answer is no. It does not affect Vmax. When there is more substrate then inhibitor present, the substrate will outcompete the inhibitor for the active site of the enzyme. Therefore, increasing the substrate concentration is a way to overcome competitive inhibition and reach the maximum target velocity. However, what changes is the substrate concentration needed to reach the maximum velocity in the presence of a competitive inhibitor. With a competitive inhibitor present, the amount of substrate needed to reach Vmax is much greater than without the inhibitor.
Uncompetitive Inhibition
Another type of enzyme inhibition is known as uncompetitive enzyme inhibition. In uncompetitive inhibition, the inhibitor does not directly compete with the substrate to bind the enzyme. The uncompetitive inhibitor cannot even bind directly to the enzyme itself. Instead, it can only bind to the enzyme-substrate complex [ES]. When it binds, it forms an enzyme-substrate-inhibitor complex, which, like in competitive inhibition, cannot react, and the reaction, therefore, is unable to proceed. Also, the uncompetitive inhibitor, unlike the competitive inhibitor, does not bind to the enzyme’s active site. It binds to a site other than the active site. In scientific terms, this site is known as the allosteric site.
In terms of KM, recall that it is inversely related to the affinity of the enzyme for the substrate. When an uncompetitive inhibitor is added, the enzyme-substrate complex will still form with the inhibitor bound to it. In this way, the concentration of enzyme [E] and substrate [S] are being used up in the reaction and will decrease overall. Therefore, the whole Michaelis-Menten saturation curve will shift to the right, which will result in more enzyme binding to the substrate. In other words, the inhibitor artificially increases the affinity of the enzyme for the substrate by combining the two with the inhibitor. As a result, then, the enzyme’s affinity for the substrate increases, which means the KM is going to decrease.
In terms of the Vmax, unlike in competitive inhibition, using extremely high substrate concentrations is unable to overcome an uncompetitive inhibitor. Adding more substrate to the reaction will result in the formation of more enzyme-substrate complexes. The enzyme-substrate complex is precisely what an uncompetitive inhibitor wants to bind. Therefore, in uncompetitive inhibition, the Vmax decreases.
Mixed Inhibition
The third type of inhibition is mixed inhibition. It is so named because it is a mix between a competitive inhibitor and an uncompetitive inhibitor. In this way, it can bind to both the free enzyme and the enzyme-substrate complex. In both cases, once the inhibitor is bound, the reaction cannot proceed. Furthermore, a mixed inhibitor binds to an allosteric site regardless of whether the enzyme has bound the substrate or not.
In terms of the effect of mixed inhibition on KM, it depends on the specific way in which the mixed inhibitor binds. A mixed inhibitor does not bind to the free enzyme and the enzyme-substrate complex with equal affinity. There are situations in which the inhibitor has a greater affinity for the free enzyme, and there are situations in which the inhibitor has a greater affinity for the enzyme-substrate complex. If the mixed inhibitor has a greater affinity for the enzyme over the enzyme-substrate complex, it is acting as a competitive inhibitor. As a result, just like in competitive inhibition, the KM will increase. If, however, the inhibitor has a higher affinity for the enzyme-substrate complex than the free enzyme, it acts more like an uncompetitive inhibitor. Just like in uncompetitive inhibition, the KM would decrease.
On the other hand, the Vmax, unlike the KM, is not variable with mixed inhibition. In mixed inhibition, the Vmax decreases. If the inhibitor can bind the enzyme-substrate complex, there is no way that a substrate can overcome the formation of this complex. Therefore, no matter what, the maximum velocity will decrease.
Noncompetitive Inhibition
Non-competitive inhibition is a subtype of mixed inhibition. In some situations, the mixed inhibitor has an equal affinity for both the free enzyme and the enzyme-substrate complex. In these instances, the mixed inhibitor acts as a non-competitive inhibitor. Since non-competitive inhibition is a form of mixed inhibition, the maximum velocity has to decrease. Furthermore, in non-competitive inhibition, the KM does not change. Even in the presence of the inhibitor, the same amount of substrate concentration is needed to get to one-half maximum reaction velocity.
Moreover, non-competitive inhibitors bind to an allosteric site. There is no competition between a substrate and the inhibitor to bind to an enzyme. In other words, in non-competitive inhibition, when an enzyme is bound to an inhibitor, it does not affect the affinity for a substrate to bind to that same enzyme. Likewise, if a substrate is bound to an enzyme, it does not affect the affinity of an inhibitor to bind to the same enzyme.
Enzyme Inhibition Summary
You should now have a good understanding of the different types of enzyme inhibition. More specifically, you should be able to explain why there are only three types of enzyme inhibition (competitive, uncompetitive, and mixed) and how noncompetitive inhibition is simply a subtype of mixed inhibition. For the MCAT, you’ll also need to understand how these inhibitors affect Michaelis Menten saturation curves and Lineweaver Burk plots!
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.