Gabriel Synthesis of Amino Acids
MCAT Biochemistry Chapter 1 - Section 3.2 - Amino Acids - Gabriel Synthesis of Amino Acids
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Amino Acids
- »
- Gabriel Synthesis of Amino Acids – MCAT Biochemistry
Sample MCAT Question - Gabriel Synthesis of Amino Acids
What is the correct sequence of steps for the Gabriel synthesis of amino acids?
I. SN2
II. Hydrolysis
III. Decarboxylation
IV. Deprotonation
a) I, II, III, IV
b) I, IV, II, III
c) IV, I, II, III
d) IV, III, IV, I
C is correct. IV, I, II, III.
In the first step of Gabriel synthesis, N-phthalimidomalonic ester is deprotonated. Next, the molecule undergoes an SN2 reaction with an alkyl halide. The third step is the hydrolysis of the protecting groups to reveal the amino group and carboxylic acids. Finally, a carboxylic acid is decarboxylated to produce the amino acid. Answer choices A, B, and D are incorrect because they present an incorrect sequence of steps.
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Gabriel Synthesis of Amino Acids
There are two methods used to synthesize α-amino acids that you need to know for the MCAT: Gabriel synthesis and Strecker synthesis. This post covers Gabriel synthesis.
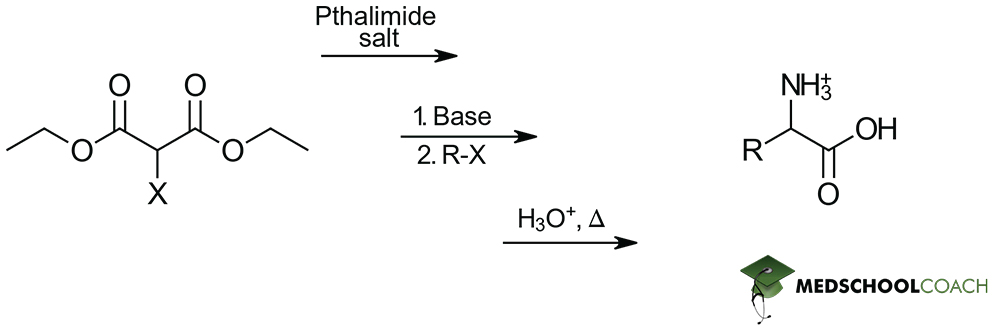
Gabriel synthesis prepares α- amino acids from N-phthalimidomalonic ester and an alkyl halide. This should seem reasonable, since the structure of N-phthalimidomalonic ester is similar to that of an amino acid attached to large protecting groups. These protecting groups are important for ensuring that the acidic carboxylic acid and the basic amine group of the structure do not react in unnecessary acid-base reactions.
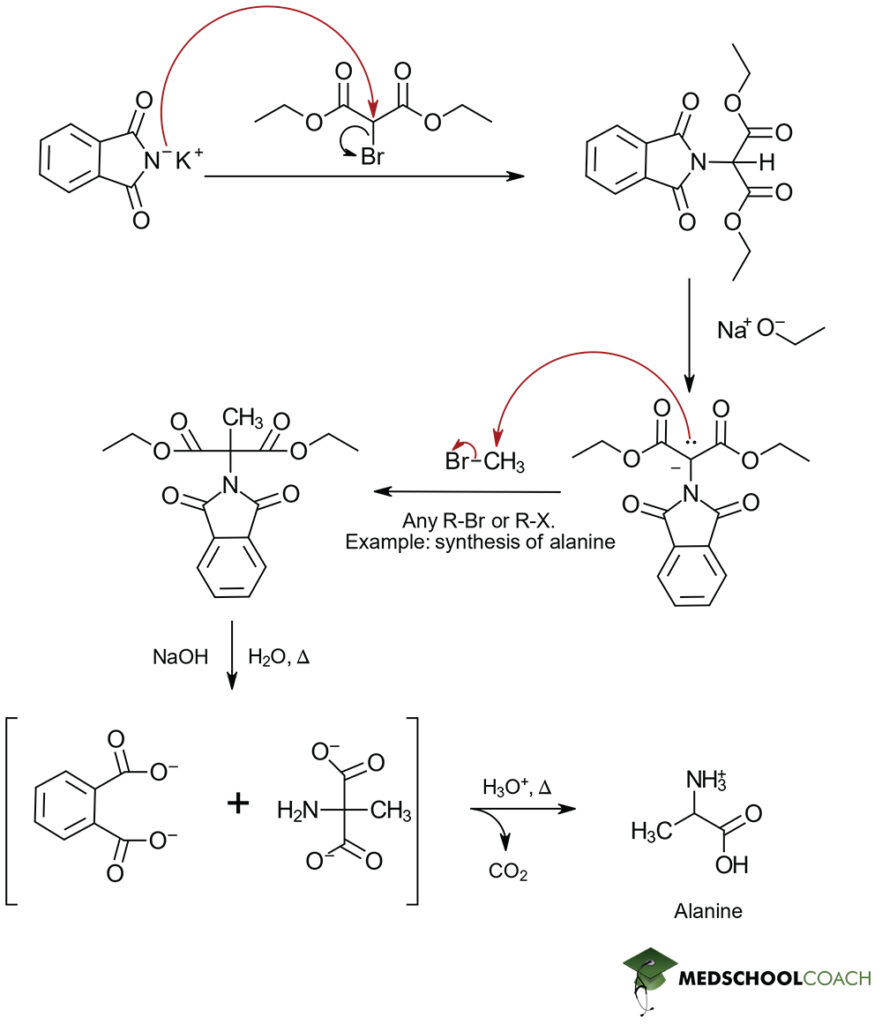
Mechanism of Gabriel Synthesis
The synthesis reaction begins with the addition of sodium ethoxide (NaOEt), a strong base to a solution of N-phthalimidomalonic ester. Sodium ethoxide removes the hydrogen atom attached to the α -carbon of N-phthalimidomalonic ester’s two ester groups, forming an enolate. When an alkyl halide is introduced, the enolate ion acts as a nucleophile, and a nucleophilic substitution reaction (specifically, an SN2 reaction) occurs. The structure of the alkyl halide determines the side chain of the amino acid. Then, a hydrolysis reaction is performed under acidic conditions. This removes the protecting groups and ester groups from around the amino group and carboxylic acid groups, respectively. The final step is introducing heat to stimulate decarboxylation, producing the α-amino acid product. Recall that decarboxylation can occur when heat is added to a compound that has two carboxylic acid groups that are β to each other.
As a final note, just like the Strecker synthesis, the Gabriel synthesis of α -amino acids produces racemic mixtures and the identity of the final α -amino acid product is entirely dependent on the nature of the R-group-containing reactant added. In this case, this addition occurs through the alkyl halide.
That covers the procedure and mechanism of Gabriel amino acid synthesis. Our other MCAT posts cover the Strecker synthesis, as well as the basic structure and stereochemistry of amino acids.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.