Electron Transport Chain
MCAT Biochemistry - Chapter 6 - Section 3.1 - Cellular Respiration - Electron Transport Chain
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Electron Transport Chain – MCAT Biochemistry
Sample MCAT Biochemistry Question
All of the following are proton pumps in the electron transport chain EXCEPT:
a) complex I.
b) complex IV.
c) complex III.
d) complex II.
D is correct. Complex II. The electron transport chain is composed of four complex transmembrane structures that are embedded in the inner membrane of the mitochondria. Three of the complexes, Complexes I, III, and IV, are electron pumps that pump protons from the mitochondrial matrix to the intermembrane space. This transfer of protons creates an electrochemical proton gradient across the inner mitochondrial membrane that is used to generate ATP. However, Complex II does not transport electrons into the intermembrane space, but instead transports electrons in a parallel fashion to Complex I. Therefore, complex II contributes less to the overall energy production of the electron transport chain.
Oxidative Phosphorylation and the Electron Transport Chain
Through glycolysis and the Krebs cycle, the cell can produce some ATP and GTP for energy. However, the bulk of energy production occurs through oxidative phosphorylation. Oxidative phosphorylation uses the energy captured by the NADH and FADH2 produced by glycolysis and the Krebs cycle to create a proton gradient that powers ATP production. Therefore, oxidative phosphorylation is a two-step process. In the first step, the electron transport chain is used to produce a proton gradient. In the second step, ATP synthase uses that proton gradient to produce ATP. This post will cover the first of these two steps: the electron transport chain.
Electron Transport Chain Overview
In the electron transport chain (ETC), the electrons from NADH and FADH2 are passed through a series of electron carriers, ultimately to oxygen, forming water. In this way, the electron transport chain is a series of oxidation reactions that function to release energy carried by NADH and FADH2. This process takes place in the inner mitochondrial membrane.
Moreover, the energy released is captured in the form of a proton gradient. As the electrons pass through electron carriers in the intermembrane space, protons are pumped from the mitochondrial matrix into the intermembrane space. Figure 1 illustrates how the ETC works. Overall, there are four complexes in the ETC: Complex I, Complex II, Complex III, and Complex IV. There are two pathways concerning these complexes. One pathway involves NADH and uses Complex I, III, and IV. The other pathway involves FADH2 and uses Complex II, III, and IV.
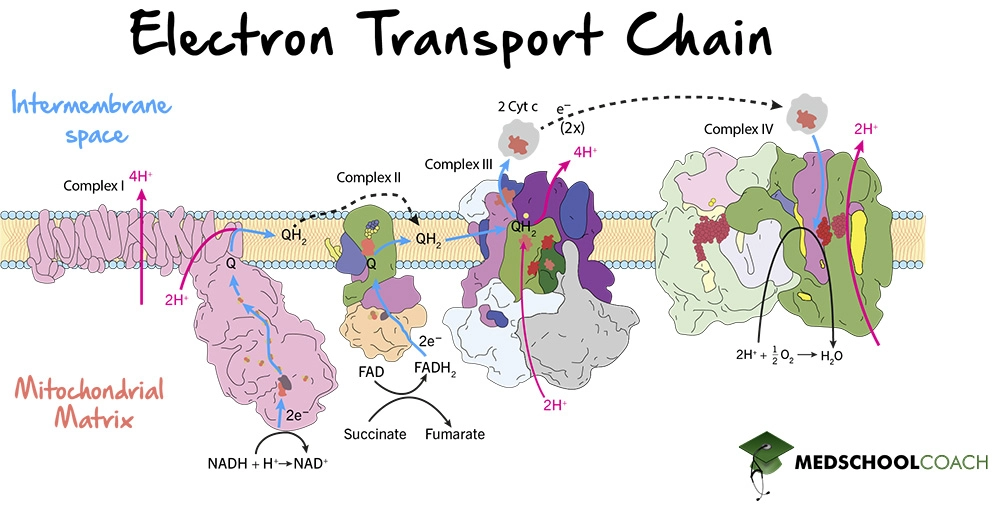
Complex I
Complex I, also known as NADH dehydrogenase, is the point at which NADH enters the ETC. Complex I oxidizes NADH to NAD+, reducing coenzyme ubiquinone to ubiquinol in a two-electron transfer. Ubiquinone is a lipid-soluble electron carrier that is also known as coenzyme Q (CoQ). Once ubiquinone is reduced to ubiquinol, ubiquinol freely diffuses within the membrane towards Complex III, and four protons from the mitochondrial matrix are pumped via Complex I into the intermembrane space. In summary, for every NADH molecule that interacts with Complex I, two electrons are transferred to CoQ and four protons are pumped to the intermembrane space.
Complex II
Complex II is also known as succinate dehydrogenase. As mentioned in the previous lesson, succinate dehydrogenase is the only enzyme that participates in both the Krebs cycle and the electron transport chain. In the Krebs cycle, succinate dehydrogenase catalyzes the oxidation of succinate to fumarate and the reduction of FAD to FADH2. In the electron transport chain, succinate dehydrogenase oxidizes FADH2 to FAD and reduces ubiquinone to ubiquinol.
In this way, both Complex I and Complex II produce ubiquinol, which then freely moves toward Complex III. Although both pathways produce the same product, the substrates they use are different. Complex I uses NADH, while Complex II uses FADH2. However, while the two molecules are different, they are both carry two electrons that are transferred to ubiquinone. Notably, however, no protons are transferred from the mitochondrial matrix into the intermembrane space by Complex II. Therefore, NADH produces more ATP per molecule than FADH2 does, because NADH interacts with Complex I which pumps protons across the membrane, while FADH2 does not contribute to proton pumping at Complex II.
Complex III
Complex III is also known as coenzyme Q: cytochrome c oxidoreductase. At this complex, ubiquinol is oxidized back to ubiquinone, and two molecules of cytochrome c (Fe3+) are reduced to cytochrome c (Fe2+). From the change in charge, it is clear that each cytochrome c molecule can only accept one electron, and is, therefore, a one-electron carrier. This means that two cytochrome c molecules per ubiquinol are necessary for this step to occur. Lastly, four protons are pumped into the intermembrane space via Complex III, further contributing to the generation of the proton gradient.
Complex IV
The last complex is Complex IV, also called cytochrome c oxidase. At this complex, two molecules of cytochrome c will be reduced from cytochrome c (Fe2+) back to cytochrome c (Fe3+). These two electrons will then be used to reduce oxygen to water. In this way, oxygen is the final electron acceptor. Also, four protons are pumped across the intermembrane space via Complex IV, contributing to the proton gradient. As mentioned previously, this proton gradient will be used by ATP synthase to produce ATP.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.
