Levels of Protein Structure
MCAT Biochemistry Chapter 2 - Section 1.1 - Peptides and Proteins - Levels of Protein Structure
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biology
- »
- Levels of Protein Structure – MCAT Biochemistry
Sample MCAT Question: Protein Structure
What level of protein structure involves hydrogen bonding from the backbone of the peptide chain?
a) Primary
b) Secondary
c) Tertiary
d) Quaternary
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Levels of Protein Structure
Proteins are polypeptides of amino acid chains that fold into various structures in order to carry out their biologic function. There are four specific levels of protein structure: primary, secondary, tertiary, and quaternary (Figure 1). Below is an outline of each structure, including the interactions that hold them together.
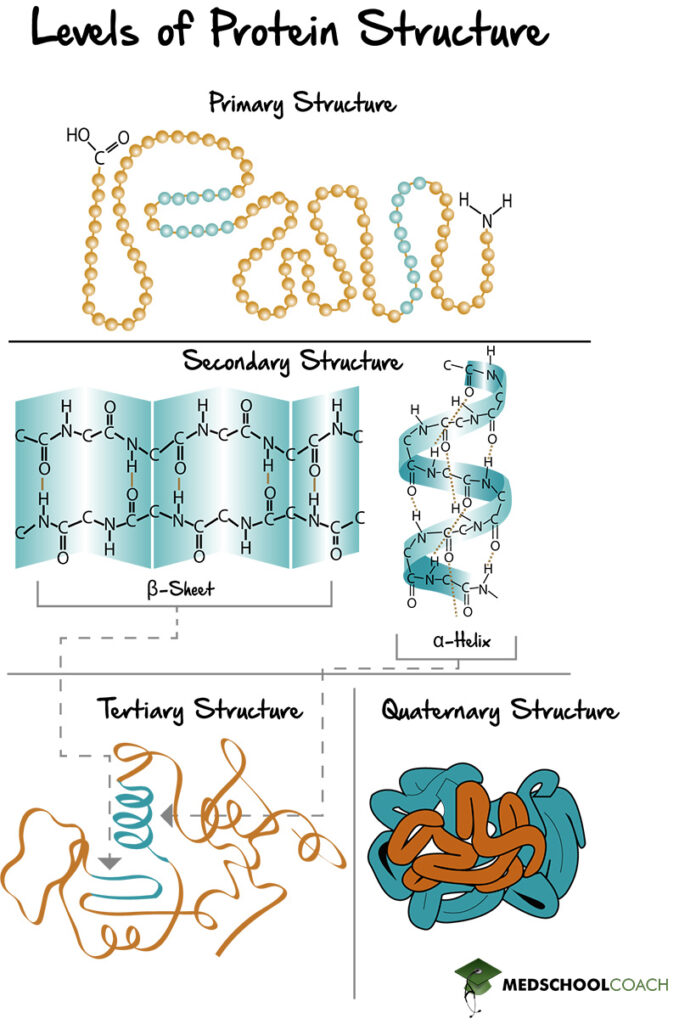
Primary Structure of Proteins
Primary protein structure is the amino acid sequence (Figure 1). In other words, it is the polypeptide chain and the specific linear sequence of the amino acids that make up a protein. In terms of amino acid chains, they are always written starting from the N-terminus and ending with the C-terminus. For example, the peptide of alanine, arginine, and glycine means that alanine is the N-terminal amino acid, and glycine is the C-terminal amino acid.
The amino acid chain is formed via peptide bonds, which join the carboxyl group of one amino acid to the amine group of the next, in a head-to-tail fashion. This formation means that the N-terminus end of the resulting protein has a free amine group, and the C-terminus end of the resulting protein has a free carboxyl group. In other words, the order of amino acids in the primary protein structure matters. A tripeptide chain of alanine, arginine, and glycine, is not the same as glycine, arginine, alanine. Lastly, peptide bonds are particularly strong because they have a partial double bond character.
Secondary Structure of Proteins (α-Helices and β-Sheets)
Secondary protein structure is the folding of the polypeptide chain to form alpha helices and beta sheets (Figure 1). Alpha helices are polypeptide chains that are held together by interactions within the chains, whereas beta-sheets are formed by different parts of the chain lining up with one another. Also, beta sheets can be parallel or antiparallel, meaning the peptide chains that are lined up with each other can be running in the same direction (parallel) or different directions (antiparallel).
Furthermore, secondary protein structures are held together by hydrogen bonds that exist between the amino hydrogen and carboxyl oxygen atoms on the backbone of the peptide chain. The amino hydrogen, or NH2 group, acts as a hydrogen bond donor, and the carboxyl oxygen has lone pairs of electrons and acts as the hydrogen bond acceptor.
Tertiary Structure of Proteins
Tertiary protein structures are formed by folding secondary protein structures together to form a globular structure (Figure 1). It is essential to understand how tertiary protein structures are formed. All proteins in the body are in an aqueous environment that is polar. The side chains of the amino acids can be polar or nonpolar. Nonpolar side chains, when folded into a tertiary structure, prefer to be buried inside the middle of the protein away from the polar solvent, exhibiting what is known as the hydrophobic effect. Dispersion forces from these hydrophobic side chains being packed tightly together help strengthen this interaction. Polar amino acids, on the other hand, tend to be exposed on the surface of the protein, interacting favorably with the polar environment.
Also, tertiary structures are held together by several different types of side-chain interactions. There are hydrogen bonds between side chains with polar groups or just general dipole interactions that do not involve hydrogen bonds. There are also hydrophobic interactions between the side chains of nonpolar amino acids. Also, salt bridges can form between an acidic amino acid and a basic amino acid. In such an interaction, an acidic amino acid donates a proton and is left with a full negative charge. If the base accepts that proton as a positive charge, this creates an amino acid with a full positive charge and one with a full negative charge. The subsequent ionic interaction between the two is known as a salt bridge. Lastly, there are disulfide bonds. All of the different types of interactions of tertiary protein structures are intermolecular forces, except the disulfide bond. Disulfide bonds are covalent interactions, which make them particularly durable.
Quaternary Structure of Proteins
The quaternary protein structure is the association of multiple polypeptide chains (Figure 1). It is important to note that not all proteins have a quaternary structure. Figure 2 shows examples of two proteins found in the human body: Myoglobin and hemoglobin. Myoglobin is made of a single polypeptide chain, meaning that it has primary, secondary, and tertiary structures. However, it does not have quaternary structure. Hemoglobin, on the other hand, is made of four different polypeptide chains called subunits. The four subunits interact with each other to form a quaternary structure that makes up the hemoglobin molecule. Furthermore, the interactions that hold a quaternary structure together are the same side-chain interactions that hold tertiary structures together. These interactions include hydrophobic interactions, dipole-dipole interactions, hydrogen bonds, salt bridges, and disulfide bonds.
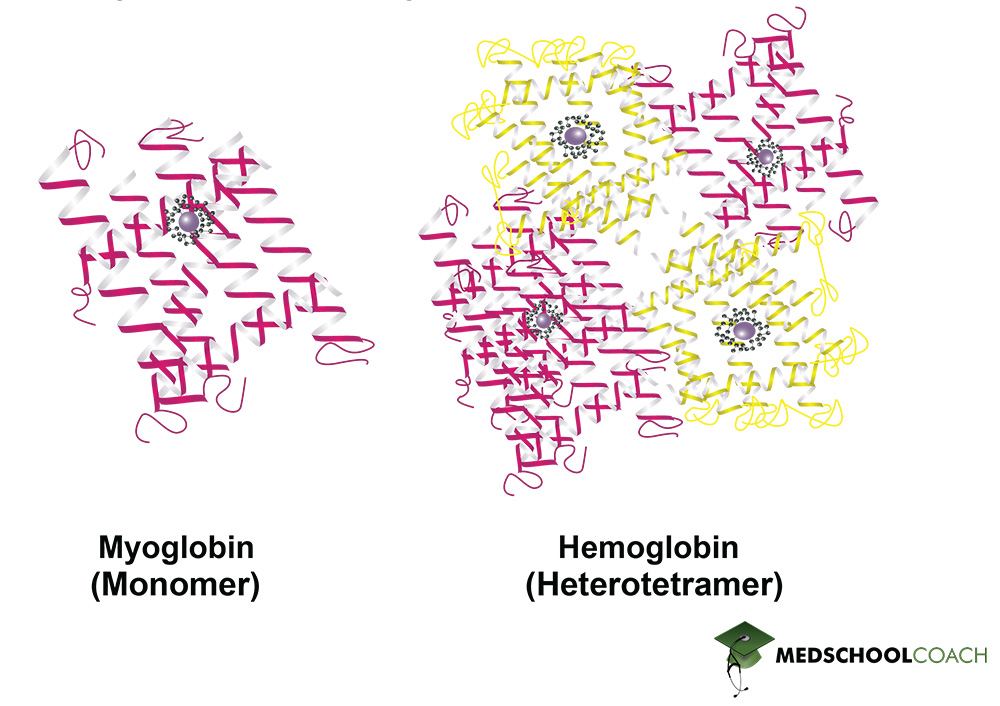
Protein Structure and Denaturation
As a final point, note that secondary, tertiary, and quaternary protein structure can be disrupted by certain conditions such as extreme temperature, pH, or salt concentrations, as well as by certain chemical agents like urea and sodium dodecyl sulfate. This is process is known as protein denaturation. Protein denaturation, as it turns out, is used to help analyze proteins in certain lab techniques, like PAGE and SDS-PAGE.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.
