Strecker Synthesis of Amino Acids
MCAT Biochemistry Chapter 1 - Section 3.1 - Amino Acids - Strecker Synthesis of Amino Acids
- Home
- »
- MCAT Masterclass
- »
- Biological and Biochemical Foundations of Living Systems
- »
- Biochemistry
- »
- Amino Acids
- »
- Strecker Synthesis of Amino Acids – MCAT Biochemistry
Sample MCAT Question - Strecker Synthesis of Amino Acids
What is the correct sequence of steps for Strecker synthesis?
I. Hydrolysis
II. Imine Formation I
II. ɑ-aminonitrile Formation
a) I, II, III
b) II, I, III
c) II, III, I
d) III, II, I
C is correct. II, III, I.
The first step of the Strecker synthesis is imine formation, where the aldehyde is converted to an imine by reacting it with a weak acid (NH4Cl). The second step is ɑ-aminonitrile formation, where a strong nucleophile (CN–) attacks the imine to form an ɑ-aminonitrile. The final step is hydrolysis, which protonates the amino group and hydrolyzes the nitrile group into a carboxyl group.
Get 1-on-1 MCAT Tutoring From a Specialist
With MCAT tutoring from MedSchoolCoach, we are committed to help you prepare, excel, and optimize your ideal score on the MCAT exam.
For each student we work with, we learn about their learning style, content knowledge, and goals. We match them with the most suitable tutor and conduct online sessions that make them feel as if they are in the classroom. Each session is recorded, plus with access to whiteboard notes. We focus on high-yield topics if you’re pressed for time. If you have more time or high-score goals, we meticulously cover the entire MCAT syllabus.
Strecker Synthesis of Amino Acids
There are two methods used to synthesize α-amino acids: Strecker synthesis and Gabriel synthesis. This post covers Strecker synthesis.
The Strecker synthesis method uses aldehydes as a starting reactant. The structure of the aldehyde determines the side chain of the synthesized amino acid. The three key steps of the Strecker synthesis are (1) imine formation, (2) α-aminonitrile formation, and (3) hydrolysis. Figure 1 provides an overview of this process.
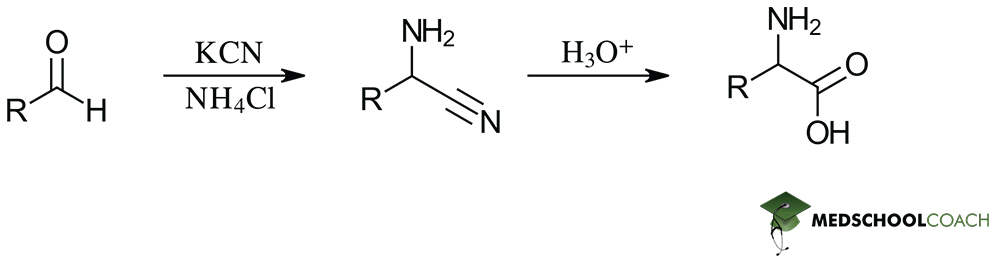
Strecker Synthesis Step 1: Imine Formation
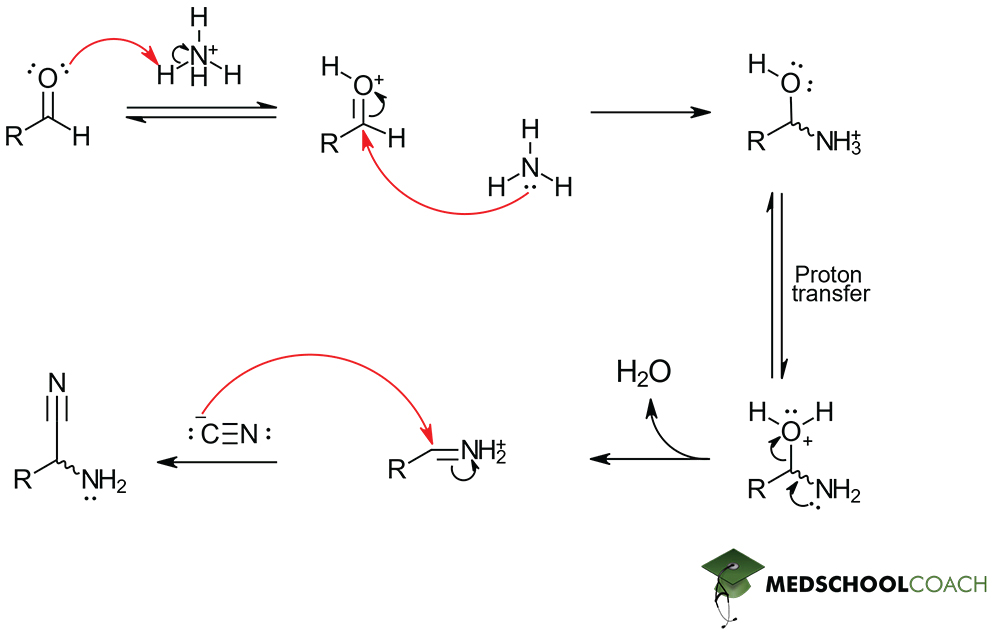
Step 1 of Strecker synthesis begins when weakly acidic ammonium protonates the aldehyde’s carbonyl .Delving into the details of this mechanism reveals to us that step 1 proceeds through protonation of the oxygen of the aldehyde’s carbonyl oxygen. This full positive charge attached to oxygen makes the carbonyl carbon an excellent electrophilic center that the remaining, nucleophilic ammonia will attack. This attack and the subsequent dehydration of the original reactant ultimately leads to imine formation.
Strecker Synthesis Step 2: α-Aminonitrile Formation
By observing the structure of imines, you will find that they are structurally similar to carbonyls. In the same way, the imine carbon is also electrophilic because nitrogen is more electronegative than carbon. Therefore, the introduction of a molecule like potassium cyanide (KCN), results in a similar electrophile-nucleophile reaction. In this step of Strecker synthesis, cyanide (CN–) acts as a nucleophile and attacks the imine carbon, resulting in the formation of an α-aminonitrile.
Strecker Synthesis Step 3: Hydrolysis
Remember that hydrolysis is simply breaking apart a compound with water. In the instance of Strecker synthesis, we have an acidic aqueous solution that hydrolyzes our nitrile group, forming a carboxylic acid and, therefore, our amino acid product.
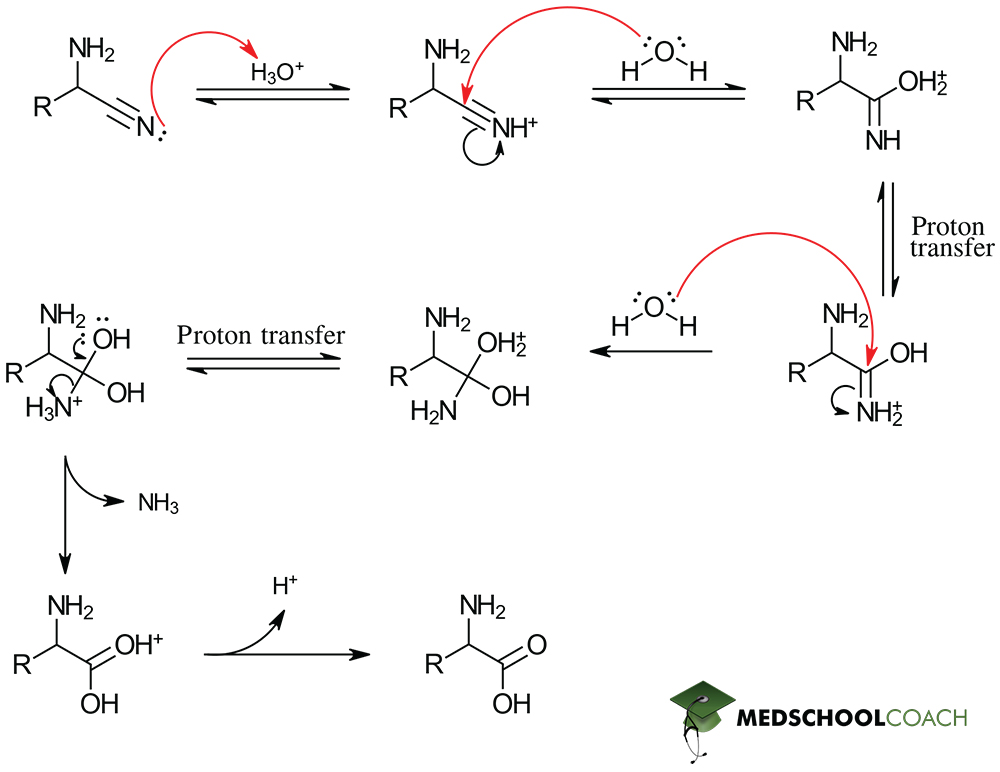
That covers the procedure and mechanism of Strecker amino acid synthesis. Our other MCAT posts cover the Gabriel amino acid synthesis as well as the basic structure and stereochemistry of amino acids.
Explore More MCAT Masterclass Chapters
Take a closer look at our entire MCAT Masterclass or explore our Biochemistry lessons below.

One-on-One Tutoring
Are you ready to take your MCAT performance to a whole new level? Work with our 99th-percentile MCAT tutors to boost your score by 12 points or more!
See if MCAT Tutoring can help me
Talk to our enrollment team about MCAT Tutoring

MCAT Go Audio Course
Engaging audio learning to take your MCAT learning on the go, any time, any where. You'll be on the way to a higher MCAT score no matter where you are. Listen to over 200+ lessons.
MCAT Practice Exams
Practice makes perfect! Our mock exams coupled with thorough explanations and in-depth analytics help students understand exactly where they stand.
MCAT Prep App
Access hundreds of MCAT videos to help you study and raise your exam score. Augment your learning with expert-created flashcards and a question banks.